New Publication in Angewandte Chemie
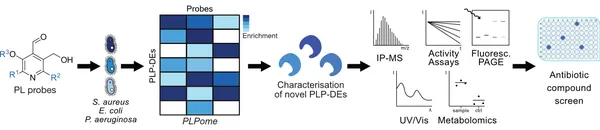
Unprecedented bacterial targets are urgently needed to overcome the resistance crisis. Herein we systematically mine pyridoxal phosphate-dependent enzymes (PLP-DEs) in bacteria to focus on a target class which is involved in crucial metabolic processes. For this, we tailored eight pyridoxal (PL) probes bearing modifications at various positions. Overall, the probes exceeded the performance of a previous generation and provided a detailed map of PLP-DEs in clinically relevant pathogens including challenging Gram-negative strains. Putative PLP-DEs with unknown function were exemplarily characterized via in-depth enzymatic assays. Finally, we screened a panel of PLP binders for antibiotic activity and unravelled the targets of hit molecules. Here, an uncharacterized enzyme, essential for bacterial growth, was assigned as PLP-dependent cysteine desulfurase and confirmed to be inhibited by the marketed drug phenelzine. Our approach provides a basis for deciphering novel PLP-DEs as essential antibiotic targets along with corresponding ways to decipher small molecule inhibitors.
Pfanzelt, M., Maher, T. E., Absmeier, R. M., Schwarz, M., Sieber, S. A.; "Tailored Pyridoxal Probes Unravel Novel Cofactor-Dependent Targets and Antibiotic Hits in Critical Bacterial Pathogens", Angew. Chem. Int. Edit.
Link: doi.org/10.1002/anie.202117724
Copyright Wiley-VCH Verlag GmbH & Co. KGaA. Reproduced with permission.