New Publication in ChemBioChem
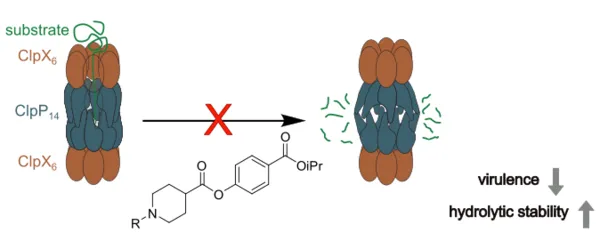
Novel strategies against multidrug-resistant bacteria are urgently needed in order to overcome the current silent pandemic. Manipulation of toxin production in pathogenic species serves as a promising approach to attenuate virulence and prevent infections. In many bacteria such as Staphylococcus aureus or Listeria monocyotgenes, serine protease ClpXP is a key contributor to virulence and thus represents a prime target for antimicrobial drug discovery. The limited stability of previous electrophilic warheads prevented a sustained effect of virulence attenuation in bacterial culture. Here, we systematically tailor the stability and inhibitory potency of phenyl ester ClpXP inhibitors by steric shielding of the ester bond and fine-tuning the phenol leaving group. Out of 17 derivatives, two (MAS-19 and MAS-30) inhibited Staphylococcus aureus ClpP peptidase and ClpXP protease activities by > 60% at 1 µM. Furthermore, the novel inhibitors did not exhibit pronounced cytotoxicity against human and bacterial cells. Unlike the first generation phenylester AV170, these molecules attenuated S. aureus virulence evidently and displayed increased stability in aqueous buffer compared to the previous benchmark AV170.
Schwarz, M., Hübner, I., Sieber, S. A., "Tailored phenyl esters inhibit ClpXP and attenuate Staphylococcus aureus α-hemolysin secretion", ChemBioChem
Link: https://doi.org/10.1002/cbic.202200253
Copyright Wiley-VCH Verlag GmbH & Co. KGaA. Reproduced with permission.