New Publication in Angewandte Chemie
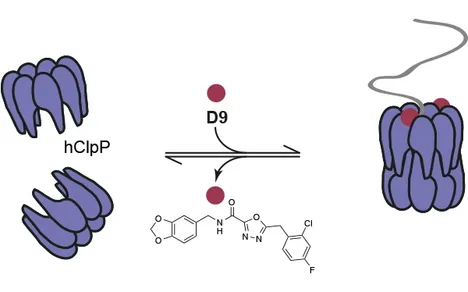
Caseinolytic protease P (ClpP) is the proteolytic component of the ClpXP protein degradation complex. Eukaryotic ClpP was recently found to act as a sensor in the mitochondria-specific unfolded protein response (UPRmt). However, its detailed function and dedicated regulation remain largely unexplored. Here we present a small molecule (D9) that acts as a potent and species-selective activator of human ClpP (hClpP) by mimicking the natural chaperone ClpX. Structure-activity relationship studies highlight the importance of a halogenated benzyl motif within D9 that interacts with a unique aromatic amino acid network in hClpP. Mutational and structural studies suggest that this YYW motif tightly controls hClpP activity and regulates substrate turnover by interaction with cognate ligands. Moreover, this signature motif is unique to ClpP from higher organisms and does not exist in bacterial homologs allowing a species-selective analysis. Thus, D9 represents a versatile tool to analyze mechanistic features of hClpP. Stahl, M., Korotkov, V.S., Balogh, D., Kick, L.M., Gersch, M., Pahl, A., Kielkowski, P., Richter, K., Schneider, S., Sieber, S.A., "Selective Activation of Human Caseinolytic Protease P (ClpP)", Angew. Chem. Int. Edit. Link: 10.1002/ange.201808189 Copyright Wiley-VCH Verlag GmbH & Co. KGaA. Reproduced with permission.