New Publication in Angewandte Chemie
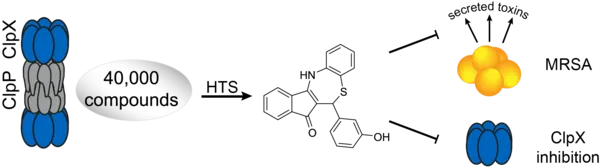
Clp its wings: A high-throughput screen against the ClpXP protease revealed the first non-covalent inhibitors of the chaperone ClpX. Binding of the inhibitors induces disruption of the ClpX hexamer and even of the whole ClpXP proteolytic complex. Treatment of Staphylococcus aureus with the compounds leads to the global down-regulation of a plethora of secreted virulence factors.
C. Fetzer, V. S. Korotkov, R. Thänert, K. M. Lee, M. Neuenschwander, J. P. von Kries, E. Medina, S. A. Sieber, "A Chemical Disruptor of the ClpX Chaperone Complex Attenuates the Virulence of Multidrug-Resistant Staphylococcus aureus", Angew. Chem. Int. Ed. 2017, 56, 15746–15750.
Link: 10.1002/anie.201708454
Copyright Wiley-VCH Verlag GmbH & Co. KGaA. Reproduced with permission.