New Review Article in Current Opinion in Chemical Biology
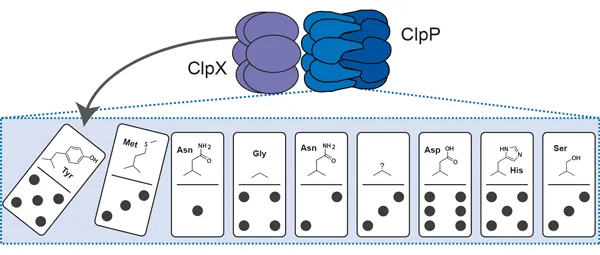
Maintaining the cellular protein homeostasis means managing life on the brink of death. This balance is largely based on precise fine-tuning of enzyme activities. For instance, the ClpP protease possesses several conformational switches which are fundamental to regulating its activity. Efforts have focused on revealing the structural basis of ClpP's conformational control. In the last decade, several amino acid clusters have been identified and functionally linked to specific activation states. Researchers have now begun to couple these hotspots to one another, uncovering a global network of residues that switch in response to internal and external stimuli. For these studies, they used small molecules to mimic intermolecular interactions and point-mutational studies to shortcut regulating amino acid circuits.
M. Stahl, S. A. Sieber, "An amino acid domino effect orchestrates ClpP’s conformational states", Curr. Opin. Chem. Biol. 2017, 40, 102–110.
doi: 10.1016/j.cbpa.2017.08.007
Copyright 2017, with permission from Elsevier.