New Publication in PNAS
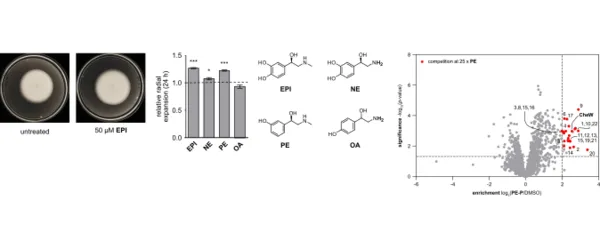
In addition to their well-known role as stress-associated catechol-amine hormones in animals and humans, epinephrine (EPI) andnorepinephrine (NE) act as interkingdom signals between eukary-otic hosts and bacteria. However, the molecular basis of theireffects on bacteria is not well understood. In initial phenotypicstudies utilizingVibrio campbelliias a model organism, we charac-terized the bipartite mode of action of catecholamines, which con-sists of promotion of growth under iron limitation and enhancedcolony expansion on soft agar. In order to identify the moleculartargets of the hormones, we designed and synthesized tailoredprobes for chemical proteomic studies. As the catechol group inEPI and NE acts as an iron chelator and is prone to form a reactivequinone moiety, we devised a photoprobe based on the adrener-gic agonist phenylephrine (PE), which solely influenced colonyexpansion. Using this probe, we identified CheW, located at thecore of the chemotaxis signaling network, as a major target. Invitro studies confirmed that EPI, NE, PE, and labetalol, a clinicallyapplied antagonist, bind to purified CheW with affinity constantsin the submicromolar range. In line with thesefindings, exposureofV. campbelliito these adrenergic agonists affects the chemotac-tic control of the bacterium. This study highlights an effect ofeukaryotic signaling molecules on bacterial motility.
Weigert Muñoz, A., Hoyer, E., Schumacher, K., Grognot, M., Taute, K. M., Hacker, S. M., Sieber, S. A.*, Jung, K.*; "Eukaryotic catecholamine hormones influence the chemotactic control of Vibrio campbellii by binding to the coupling protein CheW", PNAS
Link: doi.org/10.1073/pnas.2118227119
Copyright National Academy of Science. Reproduced with permission.