New Publication in Angewandte Chemie
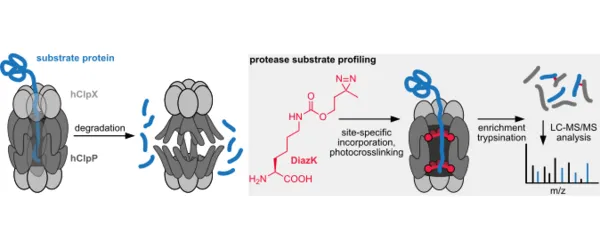
Approaches for profiling protease substrates are critical for defining protease functions, but remain challenging tasks. Here we combine genetic code expansion, photocrosslinking and proteomics to identify substrates of the mitochondrial (mt) human caseinolytic protease P (hClpP). Site-specific incorporation of the diazirine-bearing amino acid DiazK into the inner proteolytic chamber of hClpP, followed by UV-irradiation of cells allows to covalently trap hClpP to substrate proteins and to substantiate hClpP’s major involvement in maintaining overall mt homeostasis. In addition to confirming many of the previously annotated hClpP substrates, our approach adds a diverse set of new proteins to the hClpP interactome. Importantly, our workflow allows to identify substrate dynamics upon external cues in an unbiased manner. Identification of unique hClpP-substrate proteins upon induction of mt oxidative stress, suggests that hClpP counteracts oxidative stress by processing of proteins that are involved in respiratory chain complex synthesis and maturation as well as in catabolic pathways.
Nguyen, T.-A., Gronauer, T. F.*, Nast-Kolb, T., Sieber, S. A., Lang, K.; "Substrate profiling of caseinolytic protease P via a site-specificphotocrosslinking approach", Angew. Chem. Int. Edit.
Link: doi.org/10.1002/anie.202111085
Copyright Wiley-VCH Verlag GmbH & Co. KGaA. Reproduced with permission.