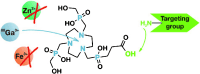
Convenient Synthesis of 68Ga-Labeled Gadolinium(III) Complexes: Towards Bimodal Responsive Probes for Functional Imaging with PET/MRI
Notni J, Hermann P, Dregely I, Wester HJ
28.08.2013 [Original Artikel]
A killer application? Recently, fully integrated full-body positron-emission tomography (PET) and magnetic-resonance imaging (MRI) scanners were brought to market, allowing simultaneous recording of complementary 3D data sets. By using bimodal PET/MRI probes (see figure), in vivo 3D mapping of various parameters with medical relevance could become feasible.
A Cyclen-Based Tetraphosphinate Chelator for the Preparation of Radiolabeled Tetrameric Bioconjugates
Šimeček J, Hermann P, Havlíčková J, Herdtweck E, Kapp TG, Engelbogen N, Kessler H, Wester HJ, Notni J
23.04.2013 [Original Artikel]
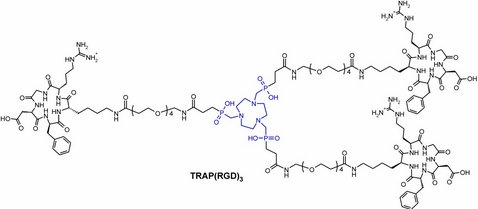
The cyclen-based tetraphosphinate chelator 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrakis[methylene(2-carboxyethyl)phosphinic acid] (DOTPI) comprises four additional carboxylic acid moieties for bioconjugation. The thermodynamic stability constants (logKML) of metal complexes, as determined by potentiometry, were 23.11 for CuII, 20.0 for LuIII, 19.6 for YIII, and 21.0 for GdIII. DOTPI was functionalized with four cyclo(Arg-Gly-Asp-D-Phe-Lys) (RGD) peptides through polyethylene glycol (PEG4) linkers. The resulting tetrameric conjugate DOTPI(RGD)4 was radiolabeled with 177Lu and 64Cu and showed improved labeling efficiency compared with 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA). The labeled compounds were fully stable in transchelation challenges against trisodium diethylenetriaminepentaacetate (DTPA) and disodium ethylenediaminetetraacetic acid (ETDA), in phosphate buffered saline (PBS), and human plasma. Integrin αvβ3 affinities of the non-radioactive LuIII and CuII complexes of DOTPI(RGD)4 were 18 times higher (both IC50 about 70 picomolar) than that of the c(RGDfK) peptide (IC50=1.3 nanomolar). Facile access to tetrameric conjugates and the possibility of radiolabeling with therapeutic and diagnostic radionuclides render DOTPI suitable for application in peptide receptor radionuclide imaging (PRRI) and therapy (PRRT).
How is 68Ga Labeling of Macrocyclic Chelators Influenced by Metal Ion Contaminants in 68Ge/68Ga Generator Eluates?
Šimeček J, Hermann P, Wester HJ, Notni J
07.11.2012 [Original Artikel]
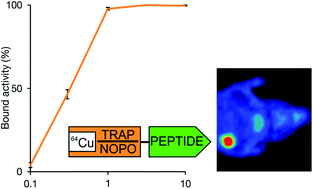
To assess the influence of Zn2+, Cu2+, Fe3+, Al3+, TiIV, and SnIV on incorporation of 68Ga3+ into pendant-arm macrocyclic chelators, the 68Ga labeling of 1,4,7-triazacyclononane-1,4,7-triacetic acid (NOTA), 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA), 1,4,7-triazacyclononane-1,4,7-tris[methyl(2-carboxyethyl)phosphinic acid]) (TRAP), and 1,4,7-triazacyclononane-1-[methyl(2-carboxyethyl)phosphinic acid]-4,7-bis[methyl(2-hydroxymethyl)phosphinic acid] (NOPO), as well as their peptide conjugates, was investigated in the presence of varying concentrations of these metal ions. The 68Ga labeling yield for carboxylate-type chelators NOTA and DOTA is decreased at lower metal ion contaminant concentrations compared with phosphinate-type chelators TRAP and NOPO. The latter are able to rapidly exchange coordinated ZnII with 68Ga3+, as confirmed by mass spectrometry and 31P NMR spectroscopy. 68Ga labeling of ZnII complexes of TRAP and NOPO proceeds as efficient as labeling of neat NOTA; this applies also to the corresponding peptide conjugates of these chelators. This behavior results in substantially improved selectivity for Ga3+ and, therefore, in more robust and reliable 68Ga labeling procedures. In addition, none of the investigated chelators binds 68Ge, rendering post-labeling purification protocols, for example, solid-phase extraction, a reliable means of removal of 68Ge contamination from 68Ga radiopharmaceuticals.
Copper-64 labelling of triazacyclononane-triphosphinate chelators
Šimeček J, Wester HJ, Notni J
08.10.2012 [Original Artikel]
The 1,4,7-triazacyclononane-1,4,7-tris(methylenephosphinic acid) chelators TRAP and NOPO are complexing copper-64 with similar efficiency as 1,4,7-triazacyclononane-triacetic acid (NOTA). The kinetic stability of Cu-64-labelled TRAP-peptides is sufficient for PET imaging at early time points (1–2 h post injection). For labelling of TRAP conjugates, Cu-64 can be recommended as an alternative to Ga-68 to achieve higher resolution of PET images.
Comparative gallium-68 labeling of TRAP-, NOTA-, and DOTA-peptides: practical consequences for the future of gallium-68-PET
Notni J, Pohle K, Wester HJ
09.06.2012 [Original Artikel]
Currently, 68Ga-labeled 1,4,7,10-tetraazacyclododecane-tetraacetic acid (DOTA)-peptides are the most widely used class of 68Ga radiotracers for PET, although DOTA is not optimal for 68Ga complexation. More recently, 1,4,7-triazacyclononane-triacetic acid (NOTA) and particularly triazacyclononane-phosphinate (TRAP) chelators have been shown to possess superior 68Ga binding ability. Here, we report on the efficiency, reproducibility, and achievable specific activity for fully automated 68Ga labeling of DOTA-, NOTA-, and TRAP-peptide conjugates. Compared to NOTA- and DOTA-peptides, achievable specific activity (A S) for TRAP-peptide is approximately 10 and 20 times higher, respectively. A S values in the range of 5,000 GBq/μmol were routinely obtained using 1 GBq of 68Ga, equivalent to 0.11 μg of cold mass for a 185-MBq patient dose of a 3-kDa conjugate. The TRAP-peptide could be 68Ga-labeled with excellent reproducibility and > 95% radiochemical yield for precursor amounts as low as 1 nmol. High 68Ga labeling efficiency of TRAP-peptides could facilitate realization of kit labeling procedures. The good reproducibility of the automated synthesis is of relevance for GMP production, and the possibility to provide very high specific activities offers a high degree of safety in first clinical trials, due to reduction of cold mass content in tracer formulations.
A Monoreactive Bifunctional Triazacyclononane Phosphinate Chelator with High Selectivity for Gallium-68
Šimeček J, Zemek O, Hermann P, Wester HJ, Notni J
12.06.2012 [Original Artikel]
Gallium-68 preferred: The macrocyclic phosphinate chelator NOPO exhibits pronounced affinity and selectivity for the Ga3+ ion, rendering it highly useful for applications in 68Ga radiopharmaceuticals for positron emission tomography.
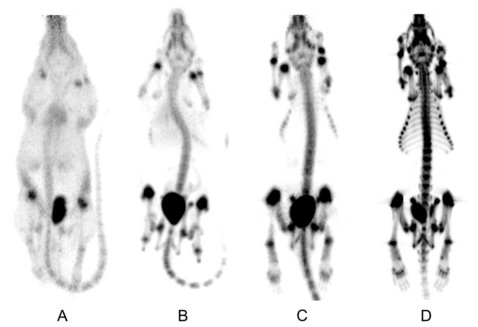
Bone-seeking TRAP conjugates: surprising observations and their implications on the development of gallium-68-labeled bisphosphonates
Notni J, Plutnar J, Wester HJ
30.03.2012 [Original Artikel]
Bisphosphonates possess strong affinity to bone. 99mTc bisphosphonate complexes are widely used for bone scintigraphy. For positron emission tomography (PET) bone imaging, Ga-68-based PET tracers based on bisphosphonates are highly desirable. Two trimeric bisphosphonate conjugates of the triazacyclononane-phosphinate (TRAP) chelator were synthesized, labeled with Ga-68, and used for microPET imaging of bone in male Lewis rats. Both Ga-68 tracers show bone uptake and, thus, are suitable for PET bone imaging. Surprisingly, Ga-71 nuclear magnetic resonance data prove that Ga(III) is not located in the chelating cavity of TRAP and must therefore be bound by the conjugated bisphosphonate units. The intrinsic Ga-68 chelating properties of TRAP are not needed for Ga-68 PET bone imaging with TRAP-bisphosphonate conjugates. Here, TRAP serves only as a trimeric scaffold. For preparation of Ga-68-based bone seekers for PET, it appears sufficient to equip branched scaffolds with multiple bisphosphonate units, which serve both Ga-68-binding and bone-targeting purposes.
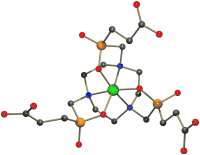
TRAP, a Powerful and Versatile Framework for Gallium-68 Radiopharmaceuticals
Notni J, Šimeček J, Hermann P, Wester HJ
06.12.2011 [Original Artikel]
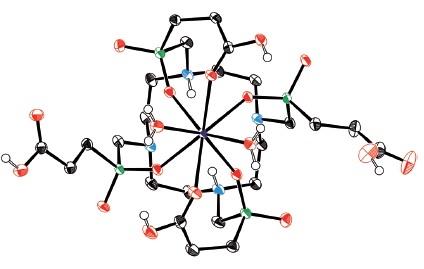
A veritable gallium TRAP: Triazacyclononane-phosphinic acid chelators (TRAP) form highly stable complexes with Ga3+ (see figure) extremely efficiently over a wide pH range. Homo- and heteromultimeric bioconjugates can be synthesized in a straightforward manner, all of which renders TRAP a chelator with ideal properties for 68Ga positron emission tomography (PET) imaging agent elaboration.