Tobias Gräwert
Bioinorganic chemistry is an interdisciplinary field at the edge of biochemistry and inorganic chemistry that deals with the function of inorganic elements in biological systems. These elements within biological systems can be seen as relics from an ancient world, where metal and mineral surfaces were the most common catalysts within a reducing atmosphere. They played a key role in the evolutionary process as they were adopted by primordial life forms. This, however, was only possible as autotrophic life evolved in an oxygen free environment, which prevented oxidation of potential metal catalysts. Yet, the circumstances changed, with the spread of photosynthetic organisms and the generation of oxygen. Nevertheless, some of these catalysts retained their importance until today and can be found in protein moieties, for example, as the metal centers in the active sites of metalloenzymes.
My main research focus has been on a subset of specialized metalloenzymes, the iron-sulfur proteins. As most of these proteins are very oxygen sensitive, extreme care has to be taken while working with them. During my postdoctoral studies in Michael Groll’s lab, I have established protocols and the environment to work under anaerobic conditions. As we worked with recombinant proteins produced in E.coli, gene expression could be performed under aerobic conditions. However, upon cell lysis, all further work steps including crystallization of proteins were performed under nitrogen atmosphere. Thus, routinely used procedures had to be adapted to the limited space inside a glove box, which especially posed a challenge concerning the growing, detection and handling of crystals. Nevertheless, we succeeded in obtaining well diffracting crystals and high resolution datasets with our in house X-ray source . Crystal trays that were stored in argon filled exsiccators also coulb be transported to synchrotron facilities like SLS or ESRF. Our aim was to to get information on the active sites of these metalloenzymes with respect to the ligand's orientation and to determine which amino acids are important for ligand binding. In some cases, however, an interdisciplinary approach in combination with other spectroscopic methods such as NMR, EPR and Mössbauer spectroscopy is necessary to fully understand the nature of a certain enzymatic reaction.
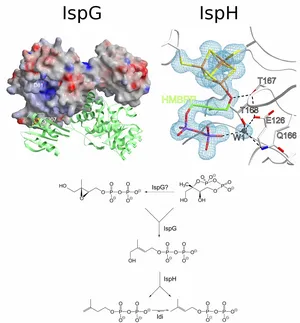
Using this approach, we were able to get valuable insights on the two iron-sulfur proteins IspG and IspH that are in particular interesting since they are essential in pathogenic organisms and can thus be considered targets. Knowledge on the reaction trajectories is important with respect to the finding of new anti-invective drugs,e.g. against Malaria or Tuberculosis. In the case of IspH, not only the active site has been elucidated with and without bound ligands at near-atomic resolutions, but also a reaction mechanism could be proposed combining crystallographic findings with results from EPR spectroscopy.
Publications
Kunfermann A., Lienau C., Illarionov B., Held J., Gräwert T., Behrendt C. T., Werner P., Hähn S., Eisenreich W., Riederer U., Mordmüller B., Bacher A., Fischer M., Groll M., Kurz T.
IspC as Target for Antiinfective Drug Discovery: Synthesis, Enantiomeric Separation, and Structural Biology of Fosmidomycin Thia Isosters
J. Med. Chem., 2013, 56, 8151–62, PDF
Span I., Gräwert T., Bacher A., Eisenreich W., Groll M.
Crystal structures of mutant IspH proteins reveal a rotation of the substrate's hydroxymethyl group during catalysis
J. Mol. Biol., 2012, 416, 1-9, PDF
Brücher K., Illarionov B., Held J., Tschan S., Kunfermann A., Pein M. K., Bacher A., Gräwert T., Maes L., Mordmüller B., Fischer M., Kurz T.
α-Substituted β-oxa isosteres of fosmidomycin: synthesis and biological evaluation.
J. Med. Chem., 2012, 55, 6566-75
Behrendt C. T., Kunfermann A., Illarionova V., Matheeussen A., Pein M. K., Gräwert T., Kaiser J., Bacher A., Eisenreich W., Illarionov B., Fischer M., Maes L., Groll M., Kurz T.
Reverse fosmidomycin derivatives against the antimalarial drug target IspC (Dxr)
J. Med. Chem., 2011, 54, 6796-802, PDF
Gräwert T., Groll M., Rohdich F., Bacher A., Eisenreich W.
Biochemistry of the non-mevalonate isoprenoid pathway
Cell Mol. Life Sci., 2011, 68, 3797-814, PDF
Lee M., Gräwert T., Quitterer F., Rohdich F., Eppinger J., Eisenreich W., Bacher A., Groll M.
Biosynthesis of isoprenoids: crystal structure of the [4Fe-4S] cluster protein IspG
J. Mol. Biol., 2010, 404, 600-10, PDF
Gräwert T., Span I., Bacher A., Groll M.
Reductive dehydroxylation of allyl alcohols by IspH protein
Angew. Chem. Int. Ed. Engl., 2010, 49, 8802-9, PDF
Behrendt C. T., Kunfermann A., Illarionova V., Matheeussen A., Gräwert T., Groll M., Rohdich F., Bacher A., Eisenreich W., Fischer M., Maes L., Kurz T.
Synthesis and antiplasmodial activity of highly active reverse analogues of the antimalarial drug candidate fosmidomycin
ChemMedChem., 2010, 5, 1673-6, PDF
Geist J. G., Lauw S., Illarionova V., Illarionov B., Fischer M., Gräwert T., Rohdich F., Eisenreich W., Kaiser J., Groll M., Scheurer C., Wittlin S., Alonso-Gómez J. L., Schweizer W. B., Bacher A., Diederich F.
Thiazolopyrimidine inhibitors of 2-methylerythritol 2,4-cyclodiphosphate synthase (IspF) from Mycobacterium tuberculosis and Plasmodium falciparum
ChemMedChem., 2010, 5, 1092-101, PDF
Gräwert T., Span I., Eisenreich W., Rohdich F., Eppinger J., Bacher A., Groll M.
Probing the reaction mechanism of IspH protein by x-ray structure analysis
Proc. Natl. Acad. Sci. USA, 2010, 107, 1077-81, PDF
Gräwert T., Rohdich F., Span I., Bacher A., Eisenreich W., Eppinger J., Groll M
Structure of active IspH enzyme provides mechanistic insights into substrate reduction
Angew. Chem. Int. Ed., 2009, 48, 5756-9, PDF