Philipp Beck
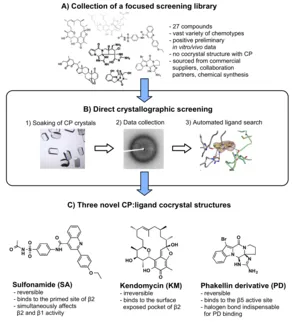
The proteasome represents a validated drug target for the treatment of cancer, however, new types of inhibitors are required to tackle the development of resistant tumors. Current fluorescence-based screening methods suffer from low sensitivity and are limited to the detection of ligands with conventional binding profiles. In my thesis, a crystallographic screening procedure for the rapid evaluation of focused compound libraries was applied to address these drawbacks (Figure 1 A/B). The optimized workflow resulted in the discovery of three unique non-peptidic reversible and irreversible proteasome ligands from natural as well as synthetic origin: i) the sulfonamide (SA), ii) the natural product kendomycin (KM) and a synthetic phakellin derivative (PD, Figure 1C). The ligands exhibit distinct chemotypes and display a hitherto unparalleled mode of action. In addition, each hit molecule populates previously unidentified CP binding sites. In summary, the described screening hits expand the druggable space that can be targeted by proteasome inhibitors. In addition, the structural insights from the presented complex structures can be exploited as starting points for the structure-based design of novel drugs with potential application for the treatment of cancer, inflammatory and autoimmune diseases as well as tool compounds for biochemical research.
During my postdoc, I focused on the evaluation of proteasome inhibitors (PIs) for targeted delivery, which could permit the treatment of solid cancers along with decreasing side effects of PIs. Thus, the first small-molecule proteasome inhibitor conjugate for targeted delivery was synthesized by fusing PIs to a synthetic ligand of somatostatin receptors, which are highly expressed in a variety of tumors. X-ray crystallographic studies and in vitro IC50 measurements demonstrated that addition of the cyclopeptide octreotide as a targeting vehicle does not affect the PI’s binding mode. The cytotoxicity of the conjugate against somatostatin-receptor-expressing cells was up to 11-fold higher than that of a non-targeting surrogate. Therefore, PIs were established as a new payload for drug conjugates and it was shown that targeted delivery thereof could be a promising approach for the broader application of this FDA-approved class of compounds.
Awards
2015: VAA Stiftungspreis für pharmazeutische Chemie for the “Entdeckung, Charakterisierung, und Synthese von neuen Proteasominhibitoren mit einem hohen Anwendungspotential”, Berlin, Germany (3,000 €)
Publications
Bräuer A., Beck P., Hintermann L., Groll M.
Structure of the Dioxygenase AsqJ: Mechanistic Insights into a One-Pot Multistep Quinolone Antibiotic Biosynthesis
Angew. Chem. Int. Ed., 2016, 55, 422-6, PDF
Beck P., Cui H., Hegemann J. D., Marahiel A. M., Krüger A., Groll M.
Targeted Delivery of Proteasome Inhibitors to Somatostatin-Receptor-Expressing Cancer Cells by Octreotide Conjugation
ChemMedChem, 2015, 10, 1969-73, PDF
Beck P., Reboud-Ravaux M., Groll M.
Identification of a β1/β2-Specific Sulfonamide Proteasome Ligand by Crystallographic Screening
Angew. Chem. Int. Ed., 2015, 54, 11275-8, PDF
Beck P., Lansdell T. A., Hewlett N. M., Tepe J. J., Groll M.
Indolo-Phakellins as β5-specific Noncovalent Proteasome Inhibitors
Angew. Chem. Int. Ed., 2015, 54, 2830–3, PDF
Voss C., Scholz C., Knorr S., Beck P., Stein M. L., Zall A., Kuckelkorn U., Kloetzel P.-M., Groll M., Hamacher K., Schmidt B.
α-Keto Phenylamides as P1'-Extended Proteasome Inhibitors
ChemMedChem, 2014, 9, 2557-64, PDF
Beck P., Heinemeyer W., Späth A.-L., Elnakady Y., Müller R., Groll M.
Interactions of the natural product kendomycin and the 20S proteasome
J. Mol. Biol., 2014, 426, 3108-17, PDF
Arciniega M., Beck P., Lange O. F., Groll M., Huber R.
Differential global structural changes in the core particle of the yeast and mouse proteasome induced by ligand binding
Proc. Natl. Acad. Sci. USA, 2014, 111, 9479-84, PDF
Quitterer F., Beck P., Bacher A., Groll M.
The formation of Pyrroline and tetrahydropyridine rings in amino acids catalyzed by pyrrolysine synthase (PylD)
Angew. Chem. Int. Ed., 2014, 53, 8150-3, PDF
Stein M. L., Cui H., Beck P., Dubiella C., Voss C., Krüger A., Schmidt B., Groll M.
Systematic Comparison of Peptidic Proteasome Inhibitors Highlights the α-Ketoamide Electrophile as an Auspicious Reversible Lead Motif
Angew. Chem. Int. Ed., 2014, 53, 1679-83, PDF
Quitterer F., Beck P., Bacher A., Groll M.
Structure and reaction mechanism of pyrrolysine synthase (PylD)
Angew. Chem. Int. Ed., 2013, 52, 7033-7, PDF
Quitterer F., List A., Beck P., Bacher A., Groll M.
Biosynthesis of the 22nd Genetically Encoded Amino Acid Pyrrolysine: Structure and Reaction Mechanism of PylC at 1.5 Å Resolution
J. Mol. Biol., 2012, 424, 270-82, PDF
Stein M. L., Beck P., Kaiser M., Dudler R., Becker C. F., Groll M.
One-shot NMR analysis of microbial secretions identifies highly potent proteasome inhibitor
Proc. Natl. Acad. Sci. USA, 2012, 109, 18367-71, PDF
Beck P., Dubiella C., Groll M.
Covalent and non-covalent reversible proteasome inhibition.
Biol. Chem. 2012, 393, 1101-20, PDF
Gallastegui N., Beck P., Arciniega M., Huber R., Hillebrand S., Groll M.
Hydroxyureas as noncovalent proteasome inhibitors
Angew. Chem. Int. Ed., 2012, 51, 247-9, PDF