Philipp Baer
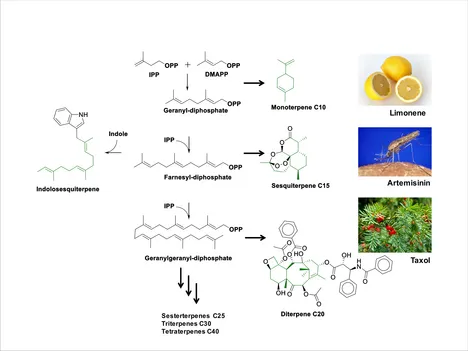
Natural products represent a major resource for pharmaceutical research. Despite their relatively small number of chemical entities compared to synthetic ones, 34% of all FDA approved drugs (1981- 2010) were based on natural products. This high biological effectivity is based on the fact, that natural products are produced by enzymes. Therefore, they target a chemical space which is capable of modulating/interacting with proteins & biological systems. With over 50,000 members, Terpenoids represent the largest class of natural products on earth. Blockbusters like the sesquiterpene Artemisinin (anti-malaria) and the diterpene Taxol (anti-cancer) are efficiently produced by engineered microbes in biotechnological setups. Strikingly, this large number of Terpenoids is mostly derived from a small number of linear polyprenyl diphosphates, including geranyl, farnesyl and geranylgeranyl diphosphates (Figure 1).
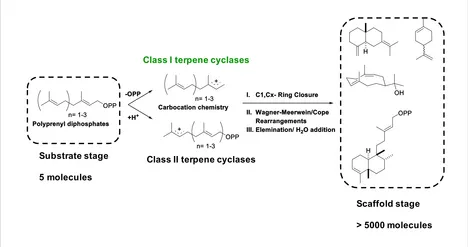
Key to this structural diversity are so-called class I/II terpene cyclases, which specifically form several carbon-carbon bonds and chiral centers within a single catalytic step. This transformation as catalyzed by class I/II terpene cyclases is among the most complex types of chemical reactions present in nature. Figure 2 summarizes this key step in terpenoid biosynthesis.
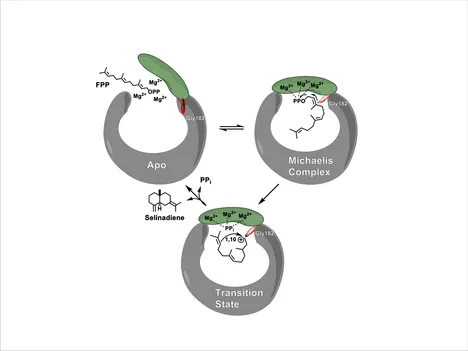
In my work as a PhD and Postdoc in the group of Michael Groll, I could contribute structural and functional insights on class I terpene cyclases’ mode of action, revealing a sophisticated induced-fit mechanism for substrate activation and highlighting the substrate’s binding mode to the active site prior to its cyclization. Crystal structures of selinadiene synthase highlighted how the induced-fit mechanism orchestrates substrate binding, active site closure and carbocation formation. This sequential reaction is of great importance, since carbocation chemistry is highly prone towards quenching with solvent molecules (H2O). The structural basis for this mechanism is a novel type of catalytic triad, the so-called effector triad. This triad comprises an arginine diphosphate sensor, a linker residue and an effector residue. The latter one’s carbonyl group shifts upon substrate binding by 5 Å, pushing its free electron pairs towards the substrate’s π*-orbital, initiating diphosphate abstraction. The crystal structure of hedycaryol synthase in complex with the reaction intermediate analog nerolidol was the first example for a class I terpene cyclase in complex with a natural ligand, whose diphosphate group is detached. Therefore, this ligand can bind deeply into the hydrophobic cavity of the active site, revealing for the first time the substrate’s exact binding mode prior to cyclization. Among other insights, our data explained how a carbonyl group of an active site residue and a helix dipole stabilize the carbocation at the thermodynamically not favorable anti-Markovnikov position, thus catalyzing the exclusive formation of the anti-Markovnikov product (Figure 3). All structural data were supported by a large set of 40 point mutants, whose product spectra were analyzed by our collaboration partner (Jeroen Dickschat, Universität Bonn).
During my Postdoc in the group of Michael Groll, in collaboration with the group of Christian Hertweck (HKI Jena), I structurally investigated a novel type of indolosesquiterpene cyclase, XiaF. A complex structure of XiaF and its oxygen-labile co-factor FADH2 provided the structural basis for explaining how nature redesigned a catabolism-derived oxygenase to use it in secondary metabolism.
Publications
Kugel S., Baunach M., Baer P., Ishida-Ito M., Sundarama S., Xua Z., Groll M., Hertweck C.
Cryptic indole hydroxylation by a non-canonical terpenoid cyclase parallels bacterial xenobiotic detoxification
Nat. Commun., 2017, 8 (15804), 1-13, PDF
Serim S., Baer P., Verhelst S. H. L.
Mixed alkyl aryl phosphonate esters as quenched fluorescent activity-based probes for serine proteases. Organic & Biomolecular Chemistry, 2015, 13, 2293-9, PDF
Baer P., Rabe P., Fischer K., Citron C. A., Klapschinski T. A., Groll M., Dickschat J. S.
Induced‐Fit Mechanism in Class I Terpene Cyclases.
Angewandte Chemie, 2014, 53, 7652-6, PDF
Baer P., Rabe P., Citron C. A., de Oliveira Mann C. C. , Kaufmann N., Groll M., Dickschat J. S.
Hedycaryol Synthase in Complex with Nerolidol Reveals Terpene Cyclase Mechanism.
ChemBioChem, 2014, 15, 213-6, PDF
Haedke U., Götz M., Baer P., Verhelst S. H.L.
Alkyne derivatives of isocoumarins as clickable activity-based probes for serine proteases.
Bioorganic & Medicinal Chemistry, 2012, 20, 633-40, PDF