Nerea Gallastegui
Proteasome inhibition has been used extensively both as marketed drugs against multiple myeloma and as valuable tool for cell biologist to fully dissect the proteasome role in protein degradation and antigen presentation. However, the toxic side effects of the currently known compounds and their unspecific binding to the different subunits of the 20S proteasome limit their potential in disease treatment and their use as research tools. We extensively studied proteasome inhibitors by using a combination of techniques, such as in-vitro fluorescent assays and in-vivo cell based assays together with the structural determination of these inhibitors in complex with the 20S proteasome in the design of novel drug.
We reported for the first time a set of linear-peptides, without any functional reactive head group, derived from the natural product TMC-95A and proceded to opitimise our lead compound into a highly Chymtrypsin-like specific inhibitor that proved to cross the cell membrane and alter a highly important pathway used in cancer treatment, that of NF-κB pathway. Other inhibitor strategies such as bivalent binding were undertaken in this work and appropriate inhibitors using an aliphatic carbon chain linker with intermediate peptide bonds, and our optimised inhibitor as a head group, were analysed.
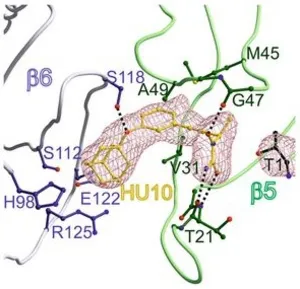
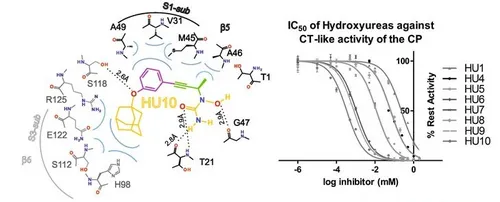
Furthermore, we discovered a new class of proteasome inhibitor that is characterized by a novel mode of ligand binding and proteasome inhibition; the hydroxyureas. The hydroxyureas represent the smallest, reversible, non-covalently bound and Chymotrypsin-like active site specific inhibitors observed to date for the 20S proteasome that do not contain any functional reactive head group. Hereby, a lead compound was discovered through the screening of a chemical library (see figure). The crystal structure of this lead compound proved to bind in a unique manner occupying pockets that so far had not been explored, the S1 and S3 sub-pockets. Combining structural research, molecular modelling, chemical synthesis and kinetic experiments we could optimise from a poor high micromolar to an optimal nanomolar range inhibitor. This work proves the strength of the combination of these techniques.
Additionally, site-specific irreversible peptide inhibitors, having an epoxyketone or vinyl sulfone functional reactive group, were characterised. Therefore, in combination these results provide an overview of 20S proteasome inhibition and delivered new insights into this highly challenging and scientifically demanding research field.
Publications
List A., Zeiler E., Gallastegui N., Rusch M, Hedberg C, Sieber S. A., Groll M.
Omuralide and Vibralactone: Differences in the Proteasome- β-Lactone-γ-Lactam Binding Scaffold Alter Target Preferences
Angew. Chem. Int. Ed., 2014, 53, 571-4, PDF
Desvergne A., Genin E., Maréchal X., Gallastegui N., Dufau L., Richy N., Groll M., Vidal J., Reboud-Ravaux M.
Dimerized linear mimics of a natural cyclopeptide (TMC-95A) are potent noncovalent inhibitors of the eukaryotic 20S proteasome
J. Med. Chem., 2013, 56, 3367-78, PDF
Geurink P. P., van der Linden W. A., Mirabella A. C., Gallastegui N., de Bruin G., Blom A. E., Voges M. J., Mock E. D., Florea B. I., van der Marel G. A., Driessen C., van der Stelt M., Groll M., Overkleeft H. S., Kisselev A. F.
Incorporation of non-natural amino acids improves cell permeability and potency of specific inhibitors of proteasome trypsin-like sites
J. Med. Chem., 2013, 56, 1262-75, PDF
Brouwer A. J., Jonker A., Werkhoven P., Kuo E., Li N., Gallastegui N., Kemmink J., Florea B. I., Groll M., Overkleeft H., Liskamp R. M.
Peptido sulfonyl fluorides as new powerful proteasome inhibitors
J. Med. Chem., 2012, 55, 10995-1003, PDF
Gallastegui N., Groll M.
Analysing properties of proteasome inhibitors using kinetic and x-ray crystallographic studies
Methods Mol. Biol., 2012, 832, 373-90, PDF
Gallastegui N., Beck P., Arciniega M., Huber R., Hillebrand S., Groll M.
Hydroxyureas as noncovalent proteasome inhibitors
Angew. Chem. Int. Ed., 2012, 51, 247-9, PDF
Gräwert M. A., Gallastegui N., Stein M. L., Schmidt B., Kloetzel P.-M., Huber R., Groll M.
Elucidation of α-Keto-Aldehyde Binding Mechanism Reveals a Novel Lead Structure Motif for Proteasome Inhibition
Angew. Chem. Int. Ed., 2011, 50, 542-4 , PDF
Groll M., Gallastegui N., Maréchal X., Le Ravalec V., Basse N., Richy N., Genin E., Huber R., Moroder L., Vidal J., Reboud-Ravaux M.
20S proteasome inhibition: designing noncovalent linear peptide mimics of the natural product TMC-95A
ChemMedChem., 2010, 5,1701-5, PDF
Gallastegui N., Groll M.
The 26S proteasome: assembly and function of a destructive machine
Trends Biochem. Sci., 2010, 35, 634-42, PDF
Gallastegui N., Groll M.
How ATPases unravel a mystery
Structure, 2009, 17, 1279-81, PDF