Maximilian Schmalhofer
Polyketides (PKs), a class of chemically complex natural products found in bacteria, fungi, and plants, often possess biological activities, making them frequently used as pharmaceuticals. My doctoral research aimed to gain insights into the biosynthesis of three distinct PKs: aryl polyene lipids (APELs), 1,8-dihydroxynaphthalene (DHN)-melanin, and anthraquinone-256 (AQ). APELs are synthesized by a novel family of type II polyketide synthase systems encoded by one of the most common biosynthetic gene clusters among Gram-negative bacteria. However, little is known about the function, biosynthesis, or biological relevance of APELs. Through X-ray crystallography, we elucidated the structures of seven homo- or heterooligomeric complexes involved in APEL biosynthesis, enabling us to characterize underlying mechanisms of catalysis, complex formation, and acyl carrier protein binding.
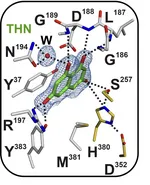
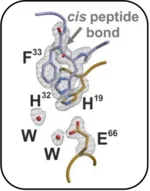
DHN-melanin, a virulence factor in pathogenic fungi, undergoes a polyketide trimming step during its biosynthesis in Aspergillus fumigatus and Exophiala dermatitidis. This carbon-carbon bond cleavage is catalyzed by the hydrolases Afyg1p and Wdyg1p. A similar cleavage reaction occurs during the biosynthesis of AQ in the pathogenic bacterium Photorhabdus laumondii, catalyzed by the homologous lyase PlAntI. We analyzed the binding and catalysis of these three enzymes using obtained X-ray crystal structures of the lyases in complex with products, surrogates, inhibitors, and compounds that mimic the sp3-hybridized intermediate state. In conclusion, our research forms the basis for future characterization of these intricate polyketide synthase systems and the design of potential inhibitors for pathogenic bacteria and fungi.

Thesis
M. Schmalhofer (2023), Mechanistic Studies on Polyketide Biosynthesis in Pathogenic Fungi and Bacteria
Publications
Su L., Schmalhofer M., Grammbitter G. L. C., Paczia N., GlatterT., Groll M., Bode H. B.
The Isopropylstilbene Precursor Cinnamic Acid Inhibits Anthraquinone Pigment Production by Targeting AntI
J. Am. Chem. Soc., 2025, 147, 20246-50, PDF
Schmalhofer M., Vagstad A. L., Zhou Q., Bode H. B., Groll M.
Polyketide trimming shapes dihydroxynaphthalene-melanin and anthraquinone pigments Polyketide trimming shapes dihydroxynaphthalene-melanin and anthraquinone pigments
Adv. Sci., 2024, 11 (e2400184), 1-10, PDF
Fraley A. E., Dell M., Schmalhofer M., Meoded R. A., Bergande C., Groll M., Piel J.
Heterocomplex structure of a polyketide synthase component involved in modular backbone halogenation
Structure, 2023, 31, 565–572, PDF
Rajeeve K., Vollmuth N., Janaki-Raman S., Wulff T. F., Baluapuri A., Dejure F. R., Huber C., Fink J., Schmalhofer M., Schmitz W., Sivadasan R., Eilers M., Wolf E., Eisenreich W., Schulze A., Seibel J., Rudel T.
Reprogramming of host glutamine metabolism during Chlamydia trachomatis infection and its key role in peptidoglycan synthesis
Nat. Microbiol., 2020, 5, 1390–1402
Bräuer A., Zhou Q., Grammbitter G. L. C., Schmalhofer M., Rühl M., Kaila V. R. I., Bode H. B., Groll M.
Structural snapshots of the minimal PKS system responsible for octaketide biosynthesis
Nat. Chem., 2020, 12, 755–763, PDF
Grammbitter G. L. C., Schmalhofer M., Karimi K., Shi Y.-M., Schöner T. A., Tobias N. J., Morgner N., Groll M., Bode H. B.
An uncommon type II PKS catalyzes biosynthesis of aryl polyene pigments
JACS, 2019, 141, 16615–16623, PDF
Zhou Q., Bräuer A., Adihou H., Schmalhofer M., Saura P., Grammbitter G. L. C., Kaila V. R. I., Groll M., Bode H. B.
Molecular mechanism of polyketide shortening in anthraquinone biosynthesis of Photorhabdus luminescens
Chem. Sci., 2019, 10, 6341–6349, PDF
Sobotta J., Schmalhofer M., Steiner T. M., Eisenreich W., Wächtershäuser G., Huber C.
One-pot formation of 2,4-di- or 2,4,6-tri-olefinic monocarboxylic acids by straight chain C4-extension
Heliyon, 2017, 3 (e00368), 1-7