Julia Hirschmann
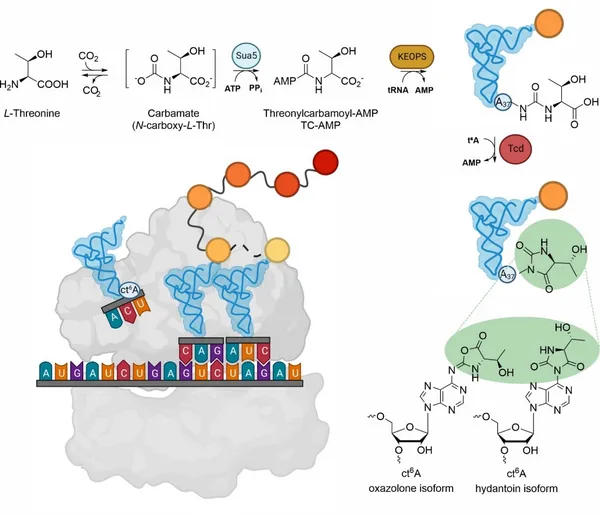
Transfer RNAs (tRNAs) are main components of protein biosynthesis in all living organisms. Serving as an adaptor molecule for decoding the genetic code, tRNA provides the required amino acids. The tRNA’s functionality and stability is fine-tuned by epigenetic modifications, also influencing its interaction with the ribosome. Each tRNA contains 13 different posttranscriptional modifications on average, often in the anticodon stem-loop region. A hotspot for modifications is the strictly conserved purine at position 37 next to the anticodon. In this work, we investigate the biosynthesis of N6-threonyl adenosine derivatives at position 37. N6-threonylcarbamoyladenosine (t6A), one of the most common modifications at this position, supports the decoding of ANN base triplets. While the enzymatic formation of t6A is well understood, the subsequent cyclization to cyclic t6A (ct6A) remains elusive. Its 3D structure - either a cyclic active ester (oxazolone) or a hydantoin - is not solved and still a matter of debate. Literature proposes threonylcarbamoyladenosine dehydratases (Tcd) as crucial players in ct6A formation, but structural and biochemical characterization is missing.
To investigate the enzyme-dependent dehydration from t6A to ct6A, we used X-ray crystallography along with various biophysical methods. Comprehensive structural and biochemical characterization, as well as a series of different purification experiments, provide valuable insights into Tcd enzymes involved in ct6A formation. Within this thesis, we discovered that the Tcd enzyme from Schizosaccharomyces pombe exists as a homodimer, while Tcd1 and Tcd2 from Saccharomyces cerevisiae form a heterodimer. Our results highlight that Tcd1 and Tcd2 from S. cerevisiae only bind tRNA when they form a heterocomplex. Tcd1 alone does not bind tRNA. Moreover, RNA extraction, sequencing, and modification analysis proved that the Tcd1Tcd2 heterocomplex interacts with tRNAs harboring a thymine at the third position of the anticodon, which matches the proposed function in decoding ANN codons in mRNAs. Additionally, in vivo tagging and subsequent Western Blot analysis successfully localized Tcd1 and Tcd2 in the outer mitochondrial membrane. These findings advance our understanding of how the essential modification t6A is further modified to ct6A.