Felix Ecker
My PhD thesis explores the biochemical and structural details of enzymatic reactions in terpene biosynthesis. This vast class of natural products comprises close to 80,000 known substances that are essential players in the biological network of life, and manifest in almost all organisms. These compounds serve critical roles as hormones, vitamins, as well as defensive agents, and are indispensable for most cellular functions. The discovery of novel terpenes with potent antibacterial, antifungal, anti-inflammatory, or immunosuppressive properties could provide promising applications in medicine and agriculture.
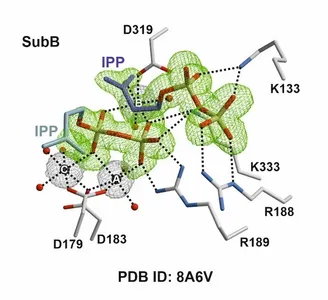
Despite the incredible diversity amongst terpenes, their origins all trace back to isomeric C5 substrates, isopentenyl (IPP), and dimethylallyl pyrophosphate (DMAPP). Short chain isoprenyl diphosphate synthases (scIDS) catalyze the initial elongation to geranyl (GPP) and farnesyl pyrophosphate (FPP). In recent years, an scIDS in the leaf beetle Phaedon cochleariae (PcIDS1) was discovered to alter its product spectrum in response to bivalent metal ions like Mg2+ and Co2+. To understand this mechanism, extensive structural analyses were combined with thermal shift assays, substrate screening, as well as mutational studies. Through over 100,000 different crystallization conditions and parameters, dozens of molecular snapshots were obtained and pieced together, revealing a sophisticated communication network. The molecular system exploits slight differences in Lewis acidity of metal ions to regulate symmetry and dynamic interactions between the molecular reaction chambers. Such a multifunctional enzyme allows the insect to rapidly adapt to changes in environmental and nutritional conditions, producing either defensive monoterpenes from GPP or utilizing FPP for hormone synthesis.
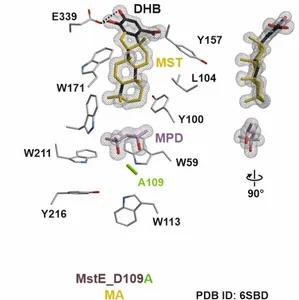
However, before they can fulfill their biological role, isoprenyl diphosphates must undergo chemical modifications, commonly through biosynthetic enzymes. In the case of steroids (derived from the eponymous cholesterol), cyclization is introduced by cyclases that are categorized into class I and II according to their domain architecture. Surprisingly, merosterolic acid synthase (MstE), identified in the cyanobacterium Scytonema, displays a so far unique type II monodomain architecture. Insights into MstE's structure reveal a unique substrate binding channel and shed light on its catalytic mechanism. Mutational studies, crystallization with natural substrates, and activity assays uncover the role of specific amino acid positions that activate and guide the nascent steroid during synthesis. Further investigations highlight potential pharmaceutical applications for meroterpenes as natural antibiotics.

Publications
Ecker F., Vattekkatte A., Boland W., Groll M.
Metal-dependent enzyme symmetry guides the biosynthetic flux of terpene precursors
Nat. Chem., 2023, 15, 1188-95 PDF
Kaspers M., Pogenberg V., Pett C., Ernst S., Ecker F., Ochtrop P., Groll M., Hedberg C., Itzen A.
Dephosphocholination by Legionella effector Lem3 functions through remodelling of the switch II region of Rab1b
Nat. Commun., 2023, 14, 1-15 PDF
Auman D., Ecker F., Mader S., Dorst K., Bräuer A., Widmalm G., Groll M., Kaila V.
Peroxy intermediate drives carbon bond activation in the dioxygenase AsqJ
J. Am. Chem. Soc., 2022, 144, 15622-32 PDF
Moosmann P., Ecker F., Leopold-Messer S., Cahn J. K. B., Dieterich C. L., Groll M., Piel J.
A monodomain class II terpene cyclase assembles complex isoprenoid scaffolds
Nat. Chem., 2020, 12, 968-972 PDF
Ernst S., Ecker F., Kaspers M. S., Ochtrop P., Hedberg C., Groll M., Itzen A.
Legionella effector AnkX displaces the switch II region for Rab1b phosphocholination
Sci. Adv., 2020, 6 (eaaz8041), 1-14, PDF
Ecker F., Haas H., Groll M., Huber E. M.
Iron scavenging in Aspergillus species: Structural and biochemical insights into fungal siderophore esterases
Angew. Chem. Int. Ed., 2018, 57 (44), 14624-9, PDF
Wachtel R., Bräuning B., Mader S., Ecker F., Kaila V., Groll M., Itzen A.
The protease GtgE from Salmonella exclusively targets inactive Rab GTPases
Nat. Commun., 2018, 9 (44), 1-13, PDF