Camille LeChapelain
I. Structural characterization of specific non-peptidic immunoproteasome inhibitors.
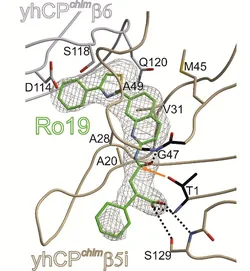
I joined a project (started by Dr. Christian Dubiella, Dr. Haissi Cui and Regina Baur) aiming at characterizing the binding mode of a new iCP inhibitor.1 The design of inhibitors specific for the immunoproteasome (iCP) represents a new avenue for the modulation of chronic inflammation and the treatment of autoimmune diseases, as they display reduced toxicity and improved pharmacokinetic properties. Furthermore, reversible inhibitors lacking an electrophilic warhead have been proven to exhibit decreased off-target reactivity, better specificity, and metabolic stability. In a recent patent, Roche Diagnostic GmbH reported a new, non-covalent iCP-specific inhibitor, Ro19, featuring a non-peptidic thiazole-substituted quinoline scaffold and displaying an IC50 in the nanomolar range. A humanized yeast proteasome mutant with a β5 subunit mimicking the human β5i subunit was employed. Analysis of the crystal structure revealed that the inhibitor does not lie in the substrate-binding channel. Thr1 forms hydrogen bonds with the carbonyl of the amide bond and the terminal carboxylic acid of Ro19. However, the cleavage of the amide bond is prevented due to the inversion of the direction of the peptide bond. The rigid aromatic moiety of Ro19 neatly occupies the S1 and S3 pockets, thus accounting for the observed β5i selectivity.
II. Development of an artificial metalloenzyme based on the streptavidin technology
In collaboration with the groups of Prof Eppinger and Prof Rueping at Kaust, we aimed at designing a new ligand comprising a biotin moiety linked to a metal center. This compound was successfully incorporated into streptavidin, and showed promising results the asymmetric transformation of alkenes. Structural characterization of the streptavidin-ligand complex is ongoing.
Publications
Zhao L., Le Chapelain C., Brachmann A. O., Kaiser M., Groll M., Bode H.B.
Activation, Structure, Biosynthesis and Bioactivity of Glidobactin-like Proteasome Inhibitors from Photorhabdus laumondi
ChemBioChem., 2021, 22 (9), 1582-88 PDF
Niroula D., Hallada L. P., Le Chapelain C., Ganegamage S. K., Dotson D., Rogelj S., Groll M. and Tello-Aburto R.
Design, synthesis, and evaluation of cystargolide-based β-lactones as potent proteasome inhibitors
Eur. J. Med. Chem., 2018, 157, 962-977, PDF
Cui H., Baur R., Le Chapelain C., Dubiella C., Heinemeyer W., Huber E. M., Groll M.
Structural Elucidation of a Nonpeptidic Inhibitor Specific for the Human Immunoproteasome
ChemBioChem., 2017, 18, 523-26, PDF
Le Chapelain C., Groll M.
Rational Design of Proteasome Inhibitors as Antimalarial Drugs
Angew. Chem. Int. Ed., 2016, 55, 6370-2, PDF
Duell E. R., Glaser M., Le Chapelain C., Antes I., Groll M., Huber E. M.
Sequential Inactivation of Gliotoxin by the S-Methyltransferase TmtA
ACS Chem. Biol., 2016, 11, 1082-9, PDF