Bastian Bräuning
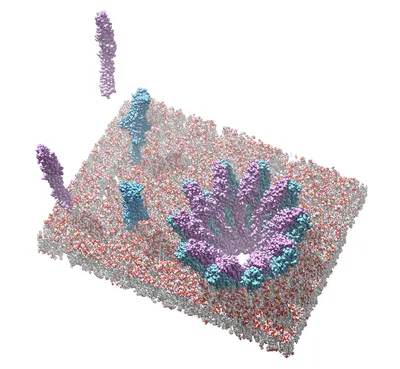
Protein channels form a passageway for water and solutes across biological lipid membranes. This property is exploited by bacterial pathogens, which secrete pore-forming toxins (PFTs) to permeabilize target cell membranes, in many cases resulting in cell death and tissue damage. Understanding the mechanism of pore assembly generally requires the structure elucidation of the soluble protein components as well as the final membrane-bound oligomeric pore. The main aim of my doctoral thesis was to gain molecular insights into lytic pore formation by the YaxAB toxin from Yersinia enterocolitica, a two-component PFT with orthologues in human, insect as well as plant pathogens. Crystal structures of the individual components YaxA and YaxB revealed unusually elongated protein topologies similar to the well-characterized ClyA family of PFTs. In close collaboration with the group of Professor Hendrik Dietz, we succeeded to obtain a cryo-electron microscopic 3D reconstruction of the reconstituted YaxAB pore, which together with structure-guided mutagenesis and activity assays, allowed us to delineate a plausible mode of action for this class of binary PFTs. These structural and mechanistic insights could fuel future efforts to exploit these toxins for biotechnological applications ranging from crop protection (using the insecticidal orthologues) to nanopore technologies. In a second branch of research, I worked towards the structure elucidation of the core pre-protein translocation complex of the outer mitochondrial membrane (TOM) from the filamentous fungi Neurospora crassa. This transmembrane protein complex is the major conduit for the passage of newly synthesized proteins across the outer membrane of this organelle. To this end I learnt and established the lipidic cubic phase (LCP) crystallization methodology for the group. LCP reconstituted TOM produced crystals that diffracted to promising resolutions, but future efforts to obtain phasing that will allow structure solution are required.
Awards
2018 – 2020: Postdoctoral stipend by the Peter und Traudl Engelhorn Foundation for “Determining GPCR lifetime at the plasma membrane from structural studies of β2 adrenergic receptor ubiquitination”, Munich, Germany
Publications
Vieweg S., Mulholland K., Bräuning B., Kachariya N., Lai Y. C., Toth R., Singh P. K., Volpi I., Sattler M., Groll M., Itzen A., Muqit M. M. K.
PINK1-dependent phosphorylation of Serine111 within the SF3 motif of Rab GTPases impairs effector interactions and LRRK2-mediated phosphorylation at Threonine72
Biochem. J., 2020, 477, 1651-68, PDF
Bräuning B., Groll M.
Structural and Mechanistic Features of ClyA-Like α-Pore-Forming Toxins
Toxins, 2018, 10 (9/343), 1-9, PDF
Bräuning B., Bertosin E., Praetorius F., Ihling C., Schatt A., Adler A., Richter K., Sinz A., Dietz H., Groll M.
Structure and mechanism of the two-component α-helical pore-forming toxin YaxAB
Nat. Commun., 2018, 9 (1806), 1-14, PDF
Wachtel R., Bräuning B., Mader S., Ecker F., Kaila V., Groll M., Itzen A.
The protease GtgE from Salmonella exclusively targets inactive Rab GTPases
Nat. Commun., 2018, 9, 1-13, PDF
Golovenko, D., Bräuning, B., Vyas, P., Haran, T. E., Rozenberg, H., Shakked, Z.
New Insights into the Role of DNA Shape on Its Recognition by p53 Proteins.
Structure, 2018, 26, 1237-1250
Solomon, H., Bräuning, B., Fainer, I., Ben-Nissan, G., Rabani, S., Goldfinger, N., Moscovitz, O., Shakked, Z., Rotter, V., Sharon, M.
Post-translational regulation of p53 function through 20S proteasome-mediated cleavage.
Cell Death Differ., 2017, 24, 2187-2198
Kolek, S. A., Bräuning, B., Stewart, P. D. S.
A novel microseeding method for the crystallization of membrane proteins in lipidic cubic phase.
Acta Crystallogr F Struct Biol Commun., 2016, 72, 307–312, PDF