Andrea Kunfermann
Nowadays, one of the most challenging problems for the treatment of infectious diseases is the rapid formation of resistances against known anti-infective or herbicides. Therefore, new chemical entities as well as new targets are in the focus of current drug research. Pseudilins, highly halogenated marine natural products, inhibit the second enzyme of the non-mevalonate pathway, IspD.
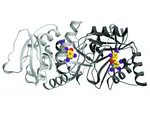
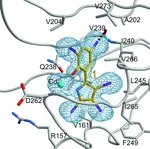
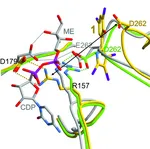
The enzymes of the non-mevalonate pathway for isoprenoid biosynthesis have been identified as attractive targets for the development of herbicides for crop protection and agents against infectious diseases. This pathway is present in many pathogenic organ- isms and plants, but absent in mammals. By using high- throughput screening, it was possible to identify the pseudilins to inhibit the third enzyme, IspD, in the pathway. Their activity against the IspD enzymes from Arabidopsis thaliana and Plasmodium vivax was determined in photometric and NMR-based assays. Crystal structures revealed that pseudilins bind to an allosteric pocket by using both divalent metal ion coordination and halogen bonding.
Pseudilins are also active against P. falciparum, a protozoan parasite causing malaria. Hereby, binding of the co-substrate cytidintriphosphatby is prevented by conformational changes, thereby locking the catalytic active site of the enzyme. However, it is still unknown whether there also exists a common regulation of the non-mevalonate pathway at the allosteric site of IspD through endogenous feedback mechanisms. This site is addressed by the pseudilin derivatives, which block the formation of the essential precursors IPP and DMAPP for terpene biosynthesis. Since halogenated marine natural products are abundant, their halogen atoms should be further investigated in biostructural studies on different targets for active participation in the molecular recognition processes such as halogen bonding.
Awards
2014: Phoenix Wissenschaftspreis, Pharmazeutische Chemie for “IspC as Target for Antiinfective Drug Discovery of Fosmidomycin Thia Isosters”, Vienna, Austria (10,000 €)
Publications
Schwab A., Illarionov B., Frank A., Kunfermann A., Seet M., Bacher A., Witschel M., Fischer M., Groll M., Diederich F.
Mechanism of Allosteric Inhibition of the Enzyme IspD by Three Different Classes of Ligands
ACS Chem. Biol., 2017, 12, 2132-8, PDF
Kunfermann A., Witschel M., Illarionov B., René M., Rottmann M., Höffken W., Seet M., Eisenreich W., Knölker H-J., Fischer M., Bacher A., Groll M., Diederich F.
Pseudilins: Halogenated, Allosteric Inhibitors of the Non-Mevalonate Pathway Enzyme IspD
Angew. Chem. Int. Ed., 2014, 53, 2235–9, PDF
Kunfermann A., Lienau C., Illarionov B., Held J., Gräwert T., Behrendt C. T., Werner P., Hähn S., Eisenreich W., Riederer U., Mordmüller B., Bacher A., Fischer M., Groll M., Kurz T.
IspC as Target for Antiinfective Drug Discovery: Synthesis, Enantiomeric Separation, and Structural Biology of Fosmidomycin Thia Isosters
J. Med. Chem., 2013, 56, 8151–62, PDF
Brücher K., Illarionov B., Held J., Tschan S., Kunfermann A., Pein M. K., Bacher A., Gräwert T., Maes L., Mordmüller B., Fischer M., Kurz T.
α-Substituted β-oxa isosteres of fosmidomycin: synthesis and biological evaluation.
J. Med. Chem., 2012, 55, 6566-75
Behrendt C. T., Kunfermann A., Illarionova V., Matheeussen A., Pein M. K., Gräwert T., Kaiser J., Bacher A., Eisenreich W., Illarionov B., Fischer M., Maes L., Groll M., Kurz T.
Reverse fosmidomycin derivatives against the antimalarial drug target IspC (Dxr)
J. Med. Chem., 2011, 54, 6796-802, PDF
Behrendt C. T., Kunfermann A., Illarionova V., Matheeussen A., Gräwert T., Groll M., Rohdich F., Bacher A., Eisenreich W., Fischer M., Maes L., Kurz T.
Synthesis and antiplasmodial activity of highly active reverse analogues of the antimalarial drug candidate fosmidomycin
ChemMedChem., 2010, 5, 1673-6, PDF
Bertz M., Kunfermann A. Rief M.
Navigating the folding energy landscape of green fluorescent protein
Angew. Chem. Int. Ed., 2008, 47, 8192-5