Alois Bräuer
The impressive range of functions and structures pre-optimized for particular biological targets and the molecular specificity are the hallmark for natural products. Biosynthetic pathways are nature’s route maps to diverse classes of natural products and an understanding of these pathways is crucial for the discovery of new and effective medicines to combat disease and overcome drug resistance. My PhD thesis gives structural and mechanistic insights into the logic of type II polyketide synthases (PKSs) at the level of protein-protein interactions. Moreover, a member of the enzyme family of FeII/α-ketoglutarate-dependent oxygenases, key tailoring and maturation catalysts in all natural product classes, was structurally and mechanistically characterized, both of vital importance for fundamental enzymology and medicinal purposes.
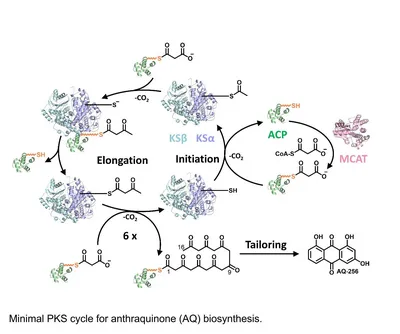
Aromatic polyketides like the antibiotic tetracycline or the anticancerogenic agent doxorubicin represent a highly important class of natural products, typically produced by type II PKSs. En route to the final compound, the acyl carrier protein (ACP) shuttles the reaction intermediates covalently attached to its prosthetic phosphopantethein (PPant) group between the different enzymatic centers of the reaction cycle. Thus, key to understand these dissociable multienzyme complexes is to characterize the ACP-partner enzyme interactions. However, due to the paradigmatic weak and transient nature of ACP-partner enzyme complexes and the instability of the poly-ß-keto intermediates only structures of individual type II PKS proteins are available so far, whereas the molecular characterization of the quaternary association is still missing in type II PKS systems. My thesis provides crystallographic insights into the complete “minimal PKS” machinery involved in anthraquinone biosynthesis from Photorhabdus luminescens, including the first complex structures between the holo-ACP (AntF) and malonyl-CoA:holo-ACP transacylase (MCAT, Plu2834) as well as the ketosynthase α/β heterodimer (KSα-KSβ, AntDE) at 3.3 Å and 2.9 Å resolution, respectively. As the “minimal PKS” core is an universal feature of PKSs, the obtained insights regarding binding sites, conformation, phosphopantethein and polyketide tunnels, as well as the role of both ketosynthase subunits, and the poly-ß-keto intermediate protection presumably apply to all PKSs. Moreover, the rare C-C bond breaking enzyme AntI has been shown to be responsible for the terminal polyketide shortening from an initial octaketide to the final heptaketide anthraquinone with its characteristic fused tricyclic ring system.
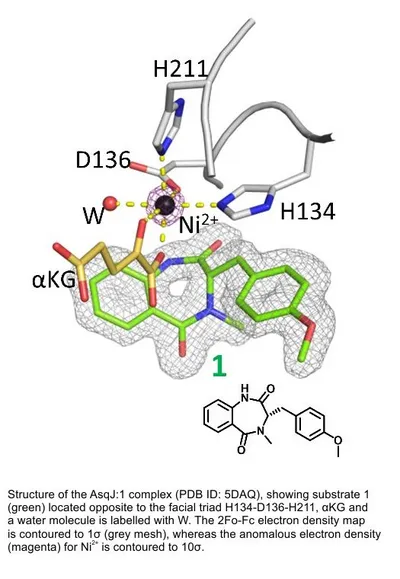
Oxygenases are key enzymes in morphing the final architectures, functional group content and polarity in all natural product classes. The FeII/α-ketoglutarate-dependent dioxygenase AsqJ from Aspergillus nidulans is outstanding because it stereoselectively catalyzes both a ferryl-induced desaturation reaction and epoxidation on a benzodiazepinedione nonribosomal peptide synthase (NRPS) product. Interestingly, the enzymatically formed spiro epoxide spring-loads the 6,7-bicyclic skeleton for non-enzymatic rearrangement into the 6,6-bicyclic scaffold of the quinolone alkaloid 4’-methoxyviridicatin. The crystal structure of the homodimeric AsqJ has been solved at atomic resolution (1.55 Å). It contains a mixed α/β-fold and forms a funnel-like reaction chamber similar to the interior of the common „jelly roll“ barrel, with the central Fe2+ coordinated octahedrically by the characteristic His-Asp-His triad, the α-ketoglutarate cofactor and a labile water molecule which is replaced by O2 to initiate the oxidation process with a high-valent FeIV-oxo species as key intermediate. Structures of the dioxygenase in the absence and presence of synthesized substrates, surrogates, and intermediates that mimic the various stages of the reaction cycle combined with HPLC/MS-coupled activity assays allowed to propose a mechanism for this unique reaction sequence. A computer-based structure-activity-relationship analysis of AsqJ revealed AsqJ-mutants with significantly improved catalytic activity for a non-methylated analog. The achieved results provide an important starting point to understand molecular mechanisms of multifunctional enzymes, and to rationally engineer such systems for the synthesis of natural products with important biomedical applications.
Publications
Auman D., Ecker F., Mader S., Dorst K., Bräuer A., Widmalm G., Groll M. and Kaila V.
Peroxy intermediate drives carbon bond activation in the dioxygenase AsqJ
J. Am. Chem. Soc., 2022, 144, 15622-32 PDF
Beaumet M., Dose A., Bräuer A., Mahy J. P., Ghattas W., Groll M and Hess C. R.
An artificial metalloprotein with metal-adaptive coordination sites and Ni-dependent quercetinase activity
J. Inorg. Biochem., 2022, 235 (111914), 1-5 PDF
Bräuer A., Zhou Q., Grammbitter G. L. C., Schmalhofer M., Rühl M., Kaila V. R. I., Bode H. B., Groll M.
Structural Snapshots of the Minimal PKS System Responsible for Octaketide Biosynthesis
Nat. Chem., 2020, 12, 755-63, PDF
Zhou Q., Bräuer A., Adihou H., Schmalhofer M., Saura P., Grammbitter G. L. C., Kaila V., Groll M., Bode H. B.
Molecular mechanism of polyketide shortening in anthraquinone biosynthesis of Photorhabdus luminescens
Chem Sci., 2019, 10, 6341-9, PDF
Mader S. L., Bräuer A., Groll M., Kaila V. R. I.
Catalytic Mechanism and Molecular Engineering of Quinolone Biosynthesis in Dioxygenase AsqJ
Nat. Commun., 2018, 9, 1-8, PDF
Bräuer A., Beck P., Hintermann L., Groll M.
Structure of the Dioxygenase AsqJ: Mechanistic Insights into a One-Pot Multistep Quinolone Antibiotic Biosynthesis
Angew. Chem. Int. Ed., 2016, 55, 422-6, PDF