Synthesis and in vitro and in vivo evaluation of urea-based PSMA inhibitors with increased lipophilicity
Wirtz M, Schmidt A, Schottelius M, Robu S, Günther T, Schwaiger M, Wester HJ
22.08.2018 [Original Artikel]
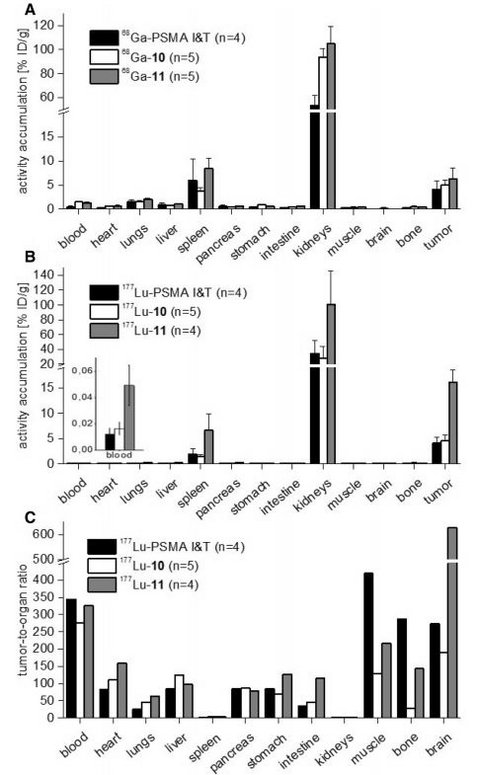
Several radiolabeled prostate-specific membrane antigen (PSMA) inhibitors based on the lysine-urea-glutamate (KuE) motif as the pharmacophore proved to be suitable tools for PET/SPECT imaging of the PSMA expression in prostate cancer patients. PSMA I&T, a theranostic tracer developed in our group, was optimized through alteration of the peptidic structure in order to increase the affinity to PSMA and internalization in PSMA-expressing tumor cells. However, further structural modifications held promise to improve the pharmacokinetic profile. Among the investigated compounds 1–9, the PSMA inhibitors 5 and 6 showed the highest PSMA affinity (lowest IC50 values) after the introduction of a naphthylalanine modification. The affinity was up to three times higher compared to the reference PSMA I&T. Extended aromatic systems such as the biphenylalanine residue in 4 impaired the interaction with the lipophilic binding pocket of PSMA, resulting in a tenfold lower affinity. The IC50 of DOTAGA-conjugated 10 was slightly increased compared to the acetylated analog; however, efficient PSMA-mediated internalization and 80% plasma protein binding of 68Ga-10 resulted in effective tumor targeting and low uptake in non-target tissues of LNCaP tumor-bearing CD-1 nu/nu mice at 1 h p.i., as determined by small-animal PET imaging and biodistribution studies. For prolonged tumor retention, the plasma protein binding was increased by insertion of 4-iodo-d-phenylalanine resulting in 97% plasma protein binding and 16.1 ± 2.5% ID/g tumor uptake of 177Lu-11 at 24 h p.i. Higher lipophilicity of the novel PSMA ligands 10 and 11 proved to be beneficial in terms of affinity and internalization and resulted in higher tumor uptake compared to the parent compound. Additional combination with para-iodo-phenylalanine in the spacer of ligand 11 elevated the plasma protein binding and enabled sustained tumor accumulation over 24 h, increasing the tumor uptake almost fourfold compared to 177Lu-PSMA I&T. However, high renal uptake remains a drawback and further studies are necessary to elucidate the responsible mechanism behind it.
Imaging Characteristics and First Experience of [68Ga]THP-PSMA, a Novel Probe for Rapid Kit-Based Ga-68 Labeling and PET Imaging: Comparative Analysis with [68Ga]PSMA I&T
Derlin T, Schmuck S, Juhl C, Teichert S, Zörgiebel J, Wester HJ, Schneefeld SM, Walte ACA, Thackeray JT, Ross TL, Bengel FM
01.08.2018 [Original Artikel]
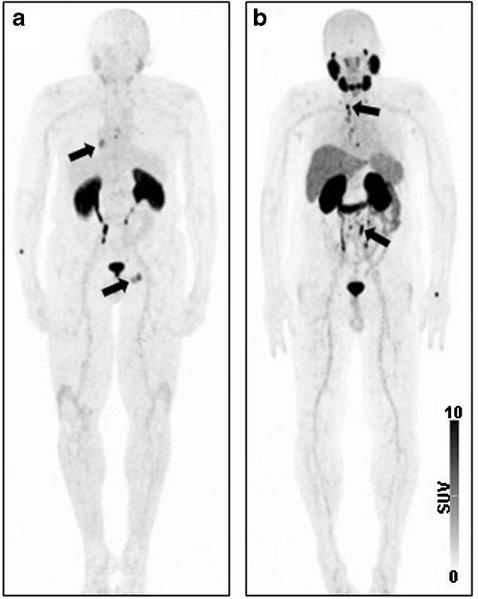
PURPOSE: [68Ga]Trishydroxypyridinone (THP)-prostate-specific membrane antigen (PSMA) is a novel tracer that can be labeled in one step by cold reconstitution of a kit with unprocessed generator eluate, targeting PSMA via the lysine-urea-glutamate (KuE) motif. The aim of this study was to evaluate the human imaging characteristics of [68Ga]THP-PSMA. PROCEDURES: [68Ga]THP-PSMA positron emission tomography (PET)/x-ray computed tomography (CT) was performed in 25 patients with biochemical recurrence after radical prostatectomy for prostate cancer. Urinary and biliary excretion and tumor lesion uptake were quantified using standardized uptake values (SUVs). Imaging characteristics were assessed in terms of non-target organ uptake, background activity, target-to-background ratios (TBRs) of tumor lesions, and frequency of bladder halo artifacts. Findings were compared to a matched cohort of 25 patients undergoing PET/CT with the established agent [68Ga]PSMA I&T. RESULTS: Physiologic uptake of [68Ga]THP-PSMA was significantly lower in salivary glands (P < 0.0001), liver (P < 0.0001), spleen (P < 0.0001), and kidneys (P < 0.0001) than with [68Ga]PSMA I&T. While biliary tracer excretion of [68Ga]THP-PSMA was negligible, urinary tracer excretion of [68Ga]THP-PSMA was fast, and significantly higher than for [68Ga]PSMA I&T, contributing to a higher frequency of bladder artifacts. Malignant lesion uptake of [68Ga]THP-PSMA assessed as either SUV or TBR was significantly lower than with [68Ga]PSMA I&T. CONCLUSION: [68Ga]THP-PSMA yields suitable in vivo uptake characteristics. The simplified synthesis method for [68Ga]THP-PSMA may facilitate wider application and higher patient throughput with PSMA imaging. However, direct intraindividual comparison studies are needed to assess the relative performance of [68Ga]THP-PSMA vs other PSMA ligands in terms of clinical detection rate and image quality.
Molar Activity of Ga-68 Labeled PSMA Inhibitor Conjugates Determines PET Imaging Results
Wurzer A, Pollmann J, Schmidt A, Reich D, Wester HJ, Notni J
27.07.2018 [Original Artikel]
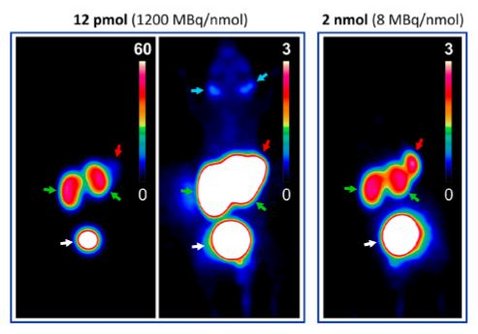
Radiopharmaceuticals targeting the enzyme prostate-specific membrane antigen (PSMA; synonyms: glutamate carboxypeptidase II, NAALADase; EC 3.4.17.21) have recently emerged as powerful agents for diagnosis and therapy (theranostics) of prostate carcinoma (PCa). The radiation doses for therapeutic application of such compounds are limited by substantial uptakes in kidneys and salivary glands, with excess doses reportedly leading to radiotoxicity-related adverse effects, such as kidney insufficiency or xenostomia. On the basis of the triazacyclononane-triphosphinate (TRAP) chelator, monomeric to trimeric conjugates of the PSMA inhibitor motif lysine-urea-glutamic acid (KuE) were synthesized by means of Cu(I)-mediated (CuAAC) or 5-aza-dibenzocyclooctyne (DBCO)-driven, strain-promoted click chemistry (SPAAC), which were labeled with gallium-68 for application in positron emission tomography (PET), and characterized in terms of PSMA affinity (determined in cellular displacement assays against I-125-BA) and lipophilicity (expressed as log D). Using subcutaneous murine LNCaP (PSMA-positive human prostate carcinoma) xenografts, the influence of ligand multiplicity, affinity, polarity, and molar activity (i.e., mass dose) on the uptakes in tumor, kidney, salivary, and background (muscle) was analyzed by means of region-of-interest (ROI) based quantification of small-animal PET imaging data. As expected, trimerization of the KuE motif resulted in high PSMA affinities (IC50 ranging from 6.0-1.5 nM). Of all parameters, molar activity/cold mass had the most pronounced influence on PET uptakes. Because accumulation in nontumor tissues was effected to a larger extent than tumor uptakes, lower molar activities resulted in substantially better tumor-to-organ ratios. For example, for one trimer, 68Ga-AhxKuE3 (IC50 = 1.5 ± 0.3 nM, log D = -3.8 ± 0.1), a higher overall amount of active compound (12 pmol vs 2 nmol, equivalent to molar activities of 1200 and 8 MBq/nmol) resulted in a remarkable reduction of the kidney-to-tumor ratio from 11.4 to 1.4, respectively, at 60 min p.i. Our study suggests that, for PSMA-targeting radiopharmaceuticals, molar activity has a more pronounced influence on small-animal PET imaging results than structural or in vitro parameters.
Effect of Carbohydration on the Theranostic Tracer PSMA I&T
Schmidt A, Wirtz M, Färber SF, Osl T, Beck R, Schottelius M, Schwaiger M, Wester HJ
25.07.2018 [Original Artikel]
To investigate the effect of carbohydrate moieties on the pharmacokinetic profile of prostate-specific membrane antigen (PSMA) inhibitors, carbohydrated derivatives of the established PSMA-targeted radiopharmaceutical PSMA I&T were developed and evaluated. As observed for the reference PSMA I&T, the natGa/natLu complexes of the respective galactose-, mannose-, and cellobiose-conjugated analogs showed high PSMA affinity. Carbohydration had almost no effect on the lipophilicity, whereas PSMA-mediated internalization was reduced. The specific binding toward human serum albumin (HSA) decreased from 78.6% for [natLu]PSMA I&T to 19.9% for the natLu-labeled cellobiose derivative. Compared to [68Ga]PSMA I&T, [68Ga]PSMA galactose displayed lower nonspecific tissue and kidney accumulation but also slightly lower tumor uptake in small-animal positron emission tomography (μPET) imaging. Biodistribution studies confirmed reduced unspecific uptake in nontarget tissue and decreased renal accumulation of the metabolically stable [68Ga]PSMA galactose derivative, resulting in overall improved tumor-to-tissue ratios. However, carbohydration has no significant beneficial in vivo effect on the targeting performance of PSMA I&T. Nevertheless, carbohydration expands the repertoire of feasible modifications within the linker area and might be a valuable tool for the future development of PSMA inhibitors with decreased kidney uptake.
Modeling and predicting tumor response in radioligand therapy
Kletting P, Thieme A, Eberhardt N, Rinscheid A, D'Alessandria C, Allmann J, Wester HJ, Tauber R, Beer AJ, Glatting G, Eiber M
10.05.2018 [Original Artikel]
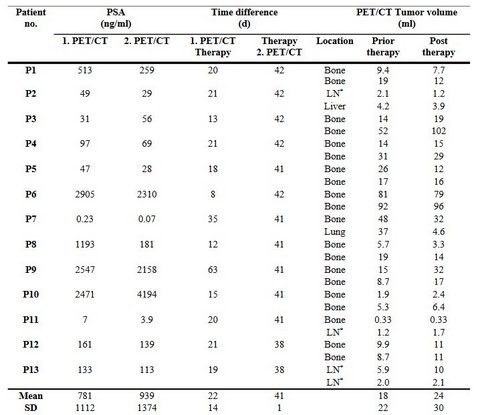
The aim of this work was to develop a theranostic method that allows predicting PSMA-positive tumor volume after radioligand therapy (RLT) based on a pre-therapeutic PET/CT measurement and physiologically based pharmacokinetic/dynamic (PBPK/PD) modeling at the example of RLT using 177Lu-labeled PSMA for imaging and therapy (PSMA I&T). Methods: A recently developed PBPK model for 177Lu PSMA I&T RLT was extended to account for tumor (exponential) growth and reduction due to irradiation (linear quadratic model). Data of 13 patients with metastatic castration-resistant prostate cancer (mCRPC) were retrospectively analyzed. Pharmacokinetic/dynamic parameters were simultaneously fitted in a Bayesian framework to PET/CT activity concentrations, planar scintigraphy data and tumor volumes prior and post (6 weeks) therapy. The method was validated using the leave-one-out Jackknife method. The tumor volume post therapy was predicted based on pre-therapy PET/CT imaging and PBPK/PD modeling. Results: The relative deviation of the predicted and measured tumor volume for PSMA-positive tumor cells (6 weeks post therapy) was 1±40% excluding one patient (PSA negative) from the population. The radiosensitivity for the PSA positive patients was determined to be 0.0172±0.0084 Gy-1. Conclusion: The proposed method is the first attempt to solely use PET/CT and modeling methods to predict the PSMA-positive tumor volume after radioligand therapy. Internal validation shows that this is feasible with an acceptable accuracy. Improvement of the method and external validation of the model is ongoing.
Synthesis and preclinical evaluation of novel 18F-labeled Glu-urea-Glu-based PSMA inhibitors for prostate cancer imaging: a comparison with 18F-DCFPyl and 18F-PSMA-1007
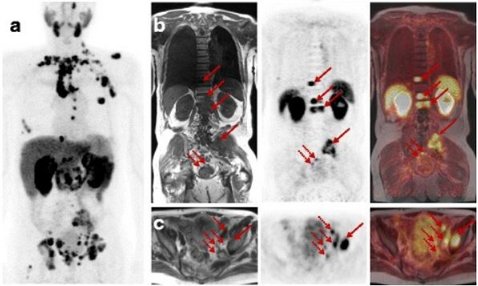
Robu S, Schmidt A, Eiber M, Schottelius M, Günther T, Hooshyar Yousefi B, Schwaiger M, Wester HJ
12.04.2018 [Original Artikel]
BACKGROUND: Due to its high and consistent expression in prostate cancer (PCa), the prostate-specific membrane antigen (PSMA) represents an ideal target for molecular imaging and targeted therapy using highly specific radiolabeled PSMA ligands. To address the continuously growing clinical demand for 18F-labeled PSMA-probes, we developed two novel Glu-urea-Glu-(EuE)-based inhibitors, EuE-k-18F-FBOA (1) and EuE-k-β-a-18F-FPyl (2), both with optimized linker structure and different 18F-labeled aromatic moieties. The inhibitors were evaluated in a comparative preclinical study with 18F-DCFPyl and 18F-PSMA-1007. RESULTS: Radiolabeling procedures allowed preparation of (1) and (2) with high radiochemical yields (67 ± 7 and 53 ± 7%, d.c.) and purity (> 98%). When compared with 18F-DCFPyl (IC50 = 12.3 ± 1.2 nM) and 18F-PSMA-1007 (IC50 = 4.2 ± 0.5 nM), both metabolically stable EuE-based ligands showed commensurable or higher PSMA affinity (IC50 = 4.2 ± 0.4 nM (1), IC50 = 1.1 ± 0.2 nM (2)). Moreover, 1.4- and 2.7-fold higher internalization rates were observed for (1) and (2), respectively, resulting in markedly enhanced tumor accumulation in LNCaP-tumor-bearing mice ((1) 12.7 ± 2.0% IA/g, (2) 13.0° ± 1.0% IA/g vs. 7.3 ± 1.0% IA/g (18F-DCFPyl), 7.1 ± 1.5% IA/g (18F-PSMA-1007), 1 h p.i.). In contrast to (1), (2) showed higher kidney accumulation and delayed clearance kinetics. Due to the high hydrophilicity of both compounds, almost no unspecific uptake in non-target tissue was observed. In contrast, due to the less hydrophilic character (logP = - 1.6) and high plasma protein binding (98%), 18F-PSMA-1007 showed uptake in non-target tissue and predominantly hepatobiliary excretion, whereas, 18F-DCFPyl exhibited pharmacokinetics quite similar to those obtained with (1) and (2). CONCLUSION: Both 18F-labeled EuE-based PSMA ligands showed excellent in vitro and in vivo PSMA-targeting characteristics. The substantially higher tumor accumulation in mice compared to recently introduced 18F-PSMA-1007 and 18F-DCFPyl suggests their high value for preclinical studies investigating the effects on PSMA-expression. In contrast to (2), (1) seems to be more promising for further investigation, due to the more reliable 18F-labeling procedure, the faster clearance kinetics with comparable high tumor uptake, resulting therefore in better high-contrast microPET imaging as early as 1 h p.i.
99mTechnetium-based Prostate-specific Membrane Antigen-radioguided Surgery in Recurrent Prostate Cancer
Maurer T, Robu S, Schottelius M, Schwamborn K, Rauscher I, van den Berg NS, van Leeuwen FWB, Haller B, Horn T, Heck MM, Gschwend JE, Schwaiger M, Wester HJ, Eiber M
03.04.2018 [Original Artikel]
BACKGROUND: Prostate-specific membrane antigen (PSMA)-targeted positron emission tomography (PET) can visualize metastatic lesions in recurrent prostate cancer (PC). However, reliable identification of small and/or atypically localized lesions during salvage surgery procedures is challenging. OBJECTIVE:To describe the technique, feasibility, and short-term outcomes of 99mTechnetium (99mTc)-based PSMA-radioguided surgery (99mTc-PSMA-RGS) for removal of recurrent PC lesions. DESIGN, SETTING, AND PARTICIPANTS: Thirty-one consecutive patients with evidence of recurrent PC on 68Ga-PSMA N,N'-bis[2-hydroxy-5-(carboxyethyl)benzyl] ethylenediamine-N,N'-diacetic acid (68Ga-PSMA-11) PET after radical prostatectomy undergoing 99mTc-PSMA-RGS were retrospectively analyzed. SURGICAL PROCEDURE: Salvage surgery with intraoperative radioguidance using a gamma probe was performed after intravenous application of 99mTc-PSMA investigation and surgery (mean activity 571 MBq, mean time to surgery 19.7h). MEASUREMENTS: Radioactive rating (positive vs negative) of resected tissue was compared with the findings of postoperative histopathological analysis. Best prostate-specific antigen (PSA) response without additional treatment was determined after 8-16 wk postoperatively. Biochemical recurrence- and treatment-free survival was evaluated. RESULTS AND LIMITATIONS: In total, 132 tissue specimens were removed, of which 58 showed metastatic involvement on histological analysis. On a specimen basis, radioactive rating yielded a sensitivity of 83.6% (confidence interval [CI]: 70.9-91.5%), a specificity of 100%, and an accuracy of 93.0% (CI: 85.5-96.7%). With 99mTc-PSMA-RGS, all lesions visualized on preoperative 68Ga-PSMA-11 PET could be removed. Moreover, 99mTc-PSMA-RGS detected additional metastases as small as 3mm in two patients. Thirteen patients suffered from complications related to surgery (Clavien-Dindo grade 1: 12 patients; grade 3a: one patient). A PSA reduction below 0.2 ng/ml was observed in 20 patients. Thirteen patients remained biochemical recurrence free after a median follow-up of 13.8 (range: 4.6-18.3) mo. Twenty patients continued to be treatment free after a median follow-up of 12.2 (range: 5.5-18.3) mo. CONCLUSIONS: As a new technique for surgical guidance, 99mTc-PSMA-RGS is feasible, and has been proved to be of high value for successful intraoperative detection and removal of metastatic lesions in PC patients scheduled for salvage surgery. Its long-term impact on outcome has to be evaluated. PATIENT SUMMARY: In this report, we evaluated a novel technique to identify metastatic lesions intraoperatively in patients with recurrent prostate cancer to facilitate surgical removal. After intravenous injection of radioactive molecules that specifically bind to prostate cancer cells that show increased expression of the prostate-specific membrane antigen, we were able to detect and remove these metastatic lesions during surgery. Following salvage surgery, 41.9% of patients remained biochemical recurrence free (median follow-up of 13.8 mo) and 64.5% continued to be treatment free (median follow-up of 12.2 mo).
Patterns of relapse as determined by 68Ga-PSMA ligand PET/CT after radical prostatectomy : Importance for tailoring and individualizing treatment
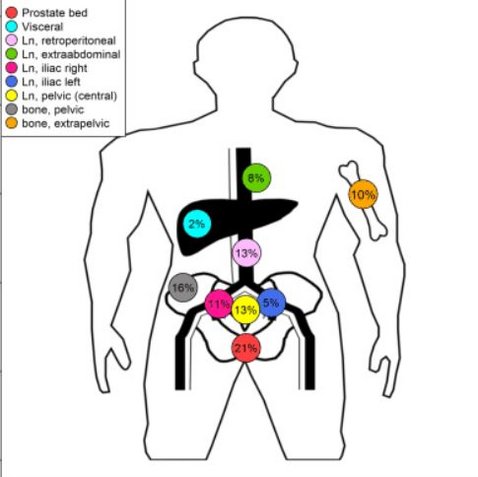
Henkenberens C, Derlin T, Bengel FM, Ross TL, Wester HJ, Hueper K, Kuczyk MA, Christiansen H, von Klot CA
01.04.2018 [Original Artikel]
PURPOSE: To evaluate the patterns of relapse and impact on the intended treatment when using 68Ga-prostate-specific membrane antigen (PSMA) ligand positron emission tomography/computed tomography (PET/CT) imaging for restaging of disease in patients with biochemical relapse after radical prostatectomy (RP) before salvage radiotherapy (sRT). METHODS: In all, 39 patients with biochemical recurrence after RP who had no primary indication for adjuvant RT due to the absence of biologically unfavorable disease (e.g., extracapsular extension, seminal vesicle invasion, positive margins, or lymph node involvement) underwent a 68Ga-PSMA ligand PET/CT for planning of sRT. RESULTS: PET/CT was positive in 84.6% (33/39) of patients. A total of 61 lesions were observed in these patients (on average 1.8 lesions per patient); 30.3% (10/33) of patients had locally recurrent disease in the prostatic bed. The clinical TNM stage (TNM: tumour-lymph nodes-metastasis-classification) was altered in 69.7% (23/33) of patients following PET, resulting in individualized treatment concepts. A prostate-specific antigen (PSA) >1.0 ng/mL was significantly associated with an increased risk of extrapelvic metastatic disease (p = 0.048). The PSA level at the time of PSMA ligand PET/CT correlated with the peak standardized uptake value (SUVpeak; p = 0.002). According to current clinical guidelines, the remaining 15.4% (6/39) of patients without evidence of disease on PET received sRT with a dose of 66.0 Gy. CONCLUSION: Our results suggest that in patients with biochemical recurrence who did not receive early sRT, a 68Ga-PSMA ligand PET/CT for restaging of disease allows for tailoring and individualizing treatment. Particularly in patients with PSA levels above 1.0 ng/mL, a 68Ga-PSMA ligand PET/CT should be performed for therapy planning, since patients often have metastases not confined to the pelvis.