Research PD Dr. Eva M. Huber
Structural basis for transcriptional regulation in fungi
The CCAAT box is a ubiquitous element of eukaryotic promoters. It is specifically bound by the heterotrimeric CCAAT-binding complex (CBC) which acts as a master regulator to control transcriptional activation and repression. In collaboration with the groups of Prof. Dr. Axel Brakhage in Jena and Prof. Dr. Hubertus Haas in Innsbruck, we investigate the structure and function of the CBC in fungi.
1. Structure of the CBC core complex
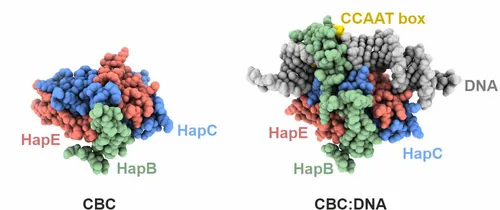
Initial work characterized the structure of the core complex alone and bound to double stranded DNA. The subunits HapC and HapE adopt a histone fold and bend the target DNA in a nucleosome-like manner. The third subunit, HapB, interacts with HapC as well as HapE and directly contacts the CCAAT motif by inserting into the minor groove. The crystallographic data explained how the CBC gains sequence specificity for the CCAAT box.
Huber, E. M., Scharf, D. H., Hortschansky, P., Groll, M., Brakhage, A. A.
DNA minor groove sensing and widening by the CCAAT-binding complex
Structure, 2012, 20 (10), 1757-68
2. Investigation of a drug resistance mechanism
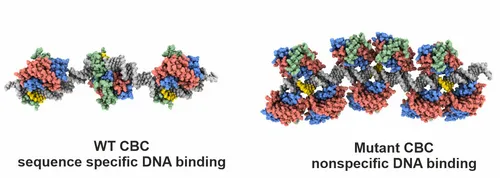
Azoles are commonly used to treat human or plant fungal infections, but resistances are on the rise. We therefore investigated how the single point mutation P88L in subunit HapE of the CBC provokes fungal azole resistance. Our X-ray structure visualized that the mutant HapE subunit interferes with the CBC’s function to bend and properly recognize its target DNA sequence, leading to transcriptional derepression of the cyp51A gene and overproduction of the 14-α sterol demethylase Cyp51A, the target of azoles.
Hortschansky, P., Misslinger, M., Mörl, Y., Gsaller, F., Bromley, M. J., Brakhage, A. A., Groll, M., Haas, H., Huber, E. M.
Structural basis of HapEP88L-linked antifungal triazole resistance in Aspergillus fumigatus
Life Sci. Alliance, 2020, 3 (7), e202000729, 1-12
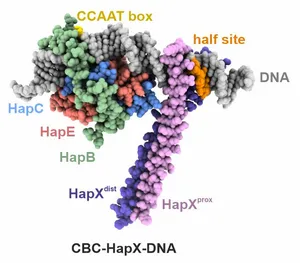
3. Cooperativity with other transcription factors
In certain fungi the CBC teams up with the dimeric basic leucine zipper HapX to control transcription of genes involved in iron metabolism. Despite detailed biochemical data, the structural basis of cooperativity between CBC and HapX remained unclear. Our crystal structure of the CBC-HapX-DNA complex now illustrates how the five-subunit assembly mediates recognition of three separate DNA motifs and how asymmetry is used to establish a 2:1 interaction between a homodimer and its binding partner.
Huber, E. M., Hortschansky, P., Scheven, M. T., Misslinger, M., Haas, H., Brakhage, A. A., Groll, M.
Structural insights into cooperative DNA recognition by the CCAAT-binding complex and its bZIP transcription factor HapX
Structure, 2022, 30(7), 934-946