Research PD Dr. Eva M. Huber
Structural aspects of epidithiodioxopiperazine biosynthesis
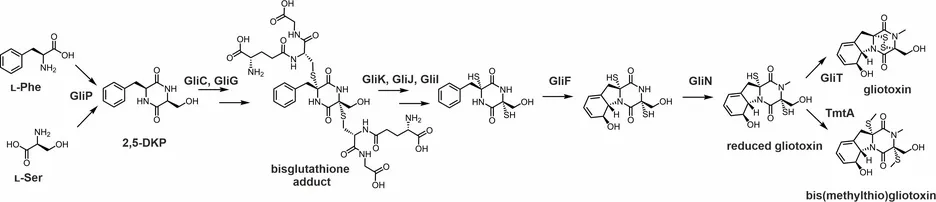
Epidithiodioxopiperazines are fungal secondary metabolites with diverse biological activities. Their 2,5-diketopiperazine scaffold, built from two amino acids, is bridged by a reactive disulfur moiety that is essential for bioactivity. In collaboration with the group of Prof. Dr. Christian Hertweck in Jena, we investigate the biosynthesis of gliotoxin, the most prominent member of this group of natural sulfur compounds. X-ray structures of key biosynthesis enzymes provide insights into ligand binding and reaction mechanisms.
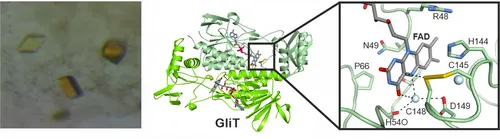
In 2014, we reported the crystal structure of the flavin adenine dinucleotide-dependent oxidoreductase GliT, which installs the disulfide bridge in gliotoxin. Together with data on two related enzymes, a unifying reaction mechanism was proposed.
Scharf, D. H., Groll, M., Habel, A., Heinekamp, T., Hertweck, C., Brakhage, A. A., Huber, E. M.
Flavoenzyme-catalyzed disulfide bridge formation in natural products
Angew. Chem. Int. Ed., 2014, 53 (8), 2221–4
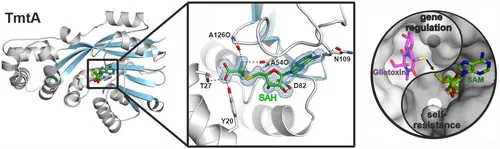
In the following, we analyzed the S-methyltransferase TmtA which uses the same substrate as GliT but converts it stepwise to bis(methylthio)gliotoxin for regulatory purposes.
Duell, E. R., Glaser, M., Le Chapelain, C., Antes, I., Groll, M., Huber, E. M.
Sequential inactivation of gliotoxin by the S-methyltransferase TmtA
ACS Chem. Biol., 2016, 11 (4), 1082–9
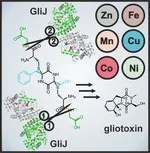
X-ray crystallography also proved a valuable method to study the dinuclear metallohydrolase GliJ. This carboxypeptidase garbles the glutathione moieties step by step and displays a surprising metal promiscuity.
Marion, A., Groll, M., Scharf, D. H., Scherlach, K., Glaser, M., Sievers, H., Schuster, M., Hertweck, C., Brakhage, A. A., Antes, I., Huber, E. M.
Gliotoxin biosynthesis: Structure, mechanism and metal promiscuity of carboxypeptidase GliJ
ACS Chem. Biol., 2017, 12 (7), 1874-1882
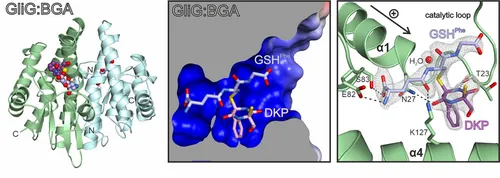
Next, we explored the mechanistic aspects of sulfur incorporation. A crystal structure of the glutathione-S-transferase GliG in complex with its reaction product, the bisglutathione adduct (BGA), suggests that the enzyme favors dehydration of the substrate and subsequently attaches glutathione. Stepwise processing of both substrate halves is required for full sulfurization.
Scherlach, K., Kuttenlochner, W., Scharf, D. H., Brakhage, A. A., Hertweck, C., Groll, M., Huber, E. M.
Structural and mechanistic insights into C-S bond formation in gliotoxin
Angew. Chem. Int. Ed., 2021, 60 (25), 14188-14194