[177Lu]pentixather: comprehensive preclinical evaluation of a first CXCR4-directed endoradiotherapeutic agent
Schottelius M, Osl T, Poschenrieder A, Hoffmann F, Beykan S, Hänscheid H, Schirbel A, Buck AK, Kropf S, Schwaiger M, Keller U, Lassmann M, Wester HJ
11.06.2017 [Original Artikel]
Based on the clinical relevance of the chemokine receptor 4 (CXCR4) as a molecular target in cancer and on the success of [68Ga]pentixafor as an imaging probe for high-contrast visualization of CXCR4-expression, the spectrum of clinical CXCR4-targeting was expanded towards peptide receptor radionuclide therapy (PRRT) by the development of [177Lu]pentixather. CXCR4 affinity, binding specificity, hCXCR4 selectivity and internalization efficiency of [177Lu]pentixather were evaluated using different human and murine cancer cell lines. Biodistribution studies (1, 6, 48, 96h and 7d p.i.) and in vivo metabolite analyses were performed using Daudi-lymphoma bearing SCID mice. Extrapolated organ doses were cross-validated with human dosimetry (pre-therapeutic and during [177Lu]pentixather PRRT) in a patient with multiple myeloma (MM). [177Lu]pentixather binds with high affinity, specificity and selectivity to hCXCR4 and shows excellent in vivo stability. Consequently, and supported by >96% plasma protein binding and a logP=-1.76, delaying whole-body clearance of [177Lu]pentixather, tumor accumulation was high and persistent, both in the Daudi model and the MM patient. Tumor/background ratios (7d p.i.) in mice were 499±202, 33±7, 4.0±0.8 and 116±22 for blood, intestine, kidney and muscle, respectively. In the patient, high tumor/kidney and tumor/liver dose ratios of 3.1 and 6.4 were observed during [177Lu]pentixather PRRT (7.8 GBq), with the kidneys being the dose-limiting organs. [177Lu]pentixather shows excellent in vivo CXCR4-targeting characteristics and a suitable pharmacokinetic profile, leading to high tumor uptake and retention and thus high radiation doses to tumor tissue during PRRT, suggesting high clinical potential of this [68Ga]pentixafor/[177Lu]pentixather based CXCR4-targeted theranostic concept.
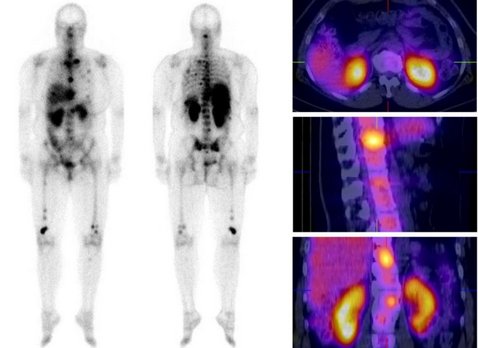
[68Ga]Pentixafor-PET/CT for imaging of chemokine receptor CXCR4 expression in multiple myeloma – Comparison to [18F]FDG and laboratory values
Constantin Lapa, Martin Schreder, Andreas Schirbel, Samuel Samnick, Klaus Martin Kortüm, Ken Herrmann, Saskia Kropf, Herrmann Einsele, Andreas K. Buck, Hans-Jürgen Wester, Stefan Knop, and Katharina Lückerath
01.01.2017 [Original Artikel]
Chemokine (C-X-C motif) receptor 4 (CXCR4) is a key factor for tumor growth and metastasis in several types of human cancer including multiple myeloma (MM). Proof-of-concept of CXCR4-directed radionuclide therapy in MM has recently been reported. This study assessed the diagnostic performance of the CXCR4-directed radiotracer [68Ga]Pentixafor in MM and a potential role for stratifying patients to CXCR4-directed therapies. Thirty-five patients with MM underwent [68Ga]Pentixafor-PET/CT for evaluation of eligibility for endoradiotherapy. In 19/35 cases, [18F]FDG-PET/CT for correlation was available. Scans were compared on a patient and on a lesion basis. Tracer uptake was correlated with standard clinical parameters of disease activity. [68Ga]Pentixafor-PET detected CXCR4-positive disease in 23/35 subjects (66%). CXCR4-positivity at PET was independent from myeloma subtypes, cytogenetics or any serological parameters and turned out as a negative prognostic factor. In the 19 patients in whom a comparison to [18F]FDG was available, [68Ga]Pentixafor-PET detected more lesions in 4/19 (21%) subjects, [18F]FDG proved superior in 7/19 (37%). In the remaining 8/19 (42%) patients, both tracers detected an equal number of lesions. [18F]FDG-PET positivity correlated with [68Ga]Pentixafor-PET positivity (p=0.018). [68Ga]Pentixafor-PET provides further evidence that CXCR4 expression frequently occurs in advanced multiple myeloma, representing a negative prognostic factor and a potential target for myeloma specific treatment. However, selecting patients for CXCR4 directed therapies and prognostic stratification seem to be more relevant clinical applications for this novel imaging modality, rather than diagnostic imaging of myeloma.
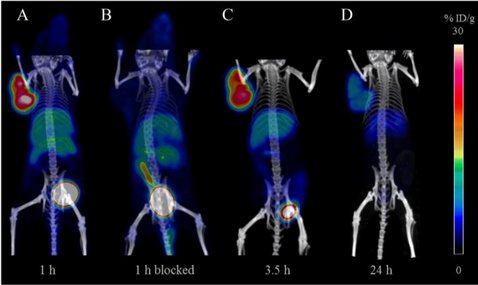
[64Cu]NOTA-pentixather enables high resolution PET imaging of CXCR4 expression in a preclinical lymphoma model
Poschenrieder A, Schottelius M, Osl T, Schwaiger M, Wester H-J
19.01.2017 [Original Artikel]
The chemokine receptor 4 (CXCR4) is an important molecular target for both visualization and therapy of tumors. The aim of the present study was the synthesis and preclinical evaluation of a 64Cu-labeled, CXCR4-targeting peptide for positron emission tomography (PET) imaging of CXCR4 expression in vivo. For this purpose, 1,4,7-triazacyclononane,1-glutaric acid-4,7-acetic acid (NODAGA), or 1,4,7-triazacyclononane-triacetic acid (NOTA) was conjugated to the highly affine CXCR4-targeting pentixather scaffold. Affinities were determined using Jurkat T-lymphocytes in competitive binding assays employing [125I]FC131 as the radioligand. Internalization and efflux studies of [64Cu]NOTA-pentixather were performed in chem-1 cells, stably transfected with hCXCR4. The stability of the tracer was evaluated in vitro and in vivo. Small-animal PET and biodistribution studies at different time points were performed in Daudi lymphoma-bearing severe combined immunodeficiency (SCID) mice. [64Cu]NOTA-pentixather was rapidly radiolabeled at 60 °C with high radiochemical yields ≥90% and purities >99%. [64Cu]NOTA-pentixather offered the highest affinity of the evaluated peptides in this study (IC50 = 14.9 ± 2.1 nM), showed efficient CXCR4-targeting in vitro and was stable in blood and urine with high resistance to transchelation in ethylenediaminetetraacetic acid (EDTA) challenge studies. Due to the enhanced lipophilicity of [64Cu]NOTA-pentixather (logP = -1.2), biodistribution studies showed some nonspecific accumulation in the liver and intestines. However, tumor accumulation (13.1 ± 1.5% ID/g, 1.5 h p.i.) was CXCR4-specific and higher than in all other organs and resulted in high resolution delineation of Daudi tumors in PET/CT images in vivo. [64Cu]NOTA-pentixather was fast and efficiently radiolabeled, showed effective CXCR4-targeting, high stability in vitro and in vivo and resulted in high resolution PET/CT images accompanied with a suitable biodistribution profile, making [64Cu]NOTA-pentixather a promising tracer for future application in humans.