Nucleotidyltransferases (NTases)
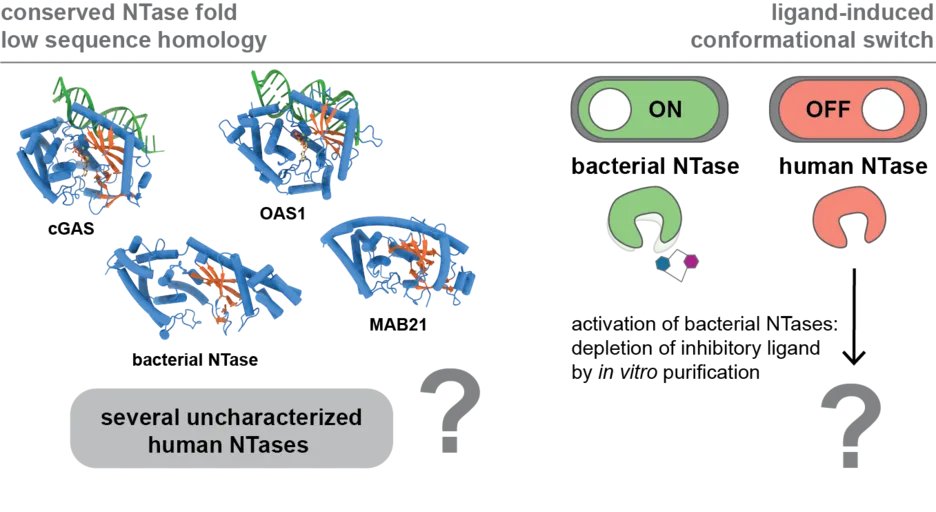
We aim to reveal the molecular function of several uncharacterized human NTases and discover novel nucleotide-based second messengers in humans. cGAS and OAS proteins belong to the large and highly diverse superfamily of nucleotidyltransferases (NTases). NTases share a common structural fold besides low sequence homology and require a ligand induced conformational switch for activation. Their common catalytic mechanism involves the transfer of nucleoside monophosphate (NMP) to a variety of substrates, including RNA/DNA, proteins, sugars, NTPs, and small molecules, with the list of potential substrates and products growing with each newly characterized NTase reaction mechanism. Several subfamilies of uncharacterized human NTases and their products remain to be discovered, despite their importance in biological processes ranging from immunity to development. Consistent with this, patient mutations in NTase family members result in drastic phenotypes and are associated to rare genetic diseases as well as cancer. Our work will be critical in revealing the molecular mechanism causing these diseases and developing novel therapeutic strategies using nucleotide-based second messengers as drugs. A challenge to reveal the molecular function of these NTases is the requirement of a yet unknown ligand for activation and product synthesis. We apply protein-nucleic acid biochemistry, structural biology using x-ray crystallography and single particle cryo-electron microscopy combined with cellular assays and small molecule mass spectrometry to identify and study NTase ligands and their products.